
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 27, No 3, May/June 2016
AFRICA
131
compared to baseline values. Among patients who developed
AKI, systolic (
p
=
0.002), diastolic (
p
=
0.023) and mean arterial
pressures (
p
=
0.003) as well as eGFR (
p
=
0.0001) values were
significantly lower on the third day of infusion compared to the
baseline value (Table 3).
Drug-related side effects were similar in both patient groups
(Table 4). A binary logistic regression analysis of co-morbidities
and drugs revealed that smoking, diastolic hypotension, and no
ASA use were significant independent predictors (
p
=
0.02,
p
=
0.003 and
p
=
0.008, respectively) for the development of AKI
during iloprost treatment.
We also evaluated factors associated with 30-day mortality
and compared survival ratios between the patient groups. The
Cox regression analysis revealed that diabetes mellitus (
p
=
0.005)
and AKI (
p
=
0.012) are significant determinants of mortality
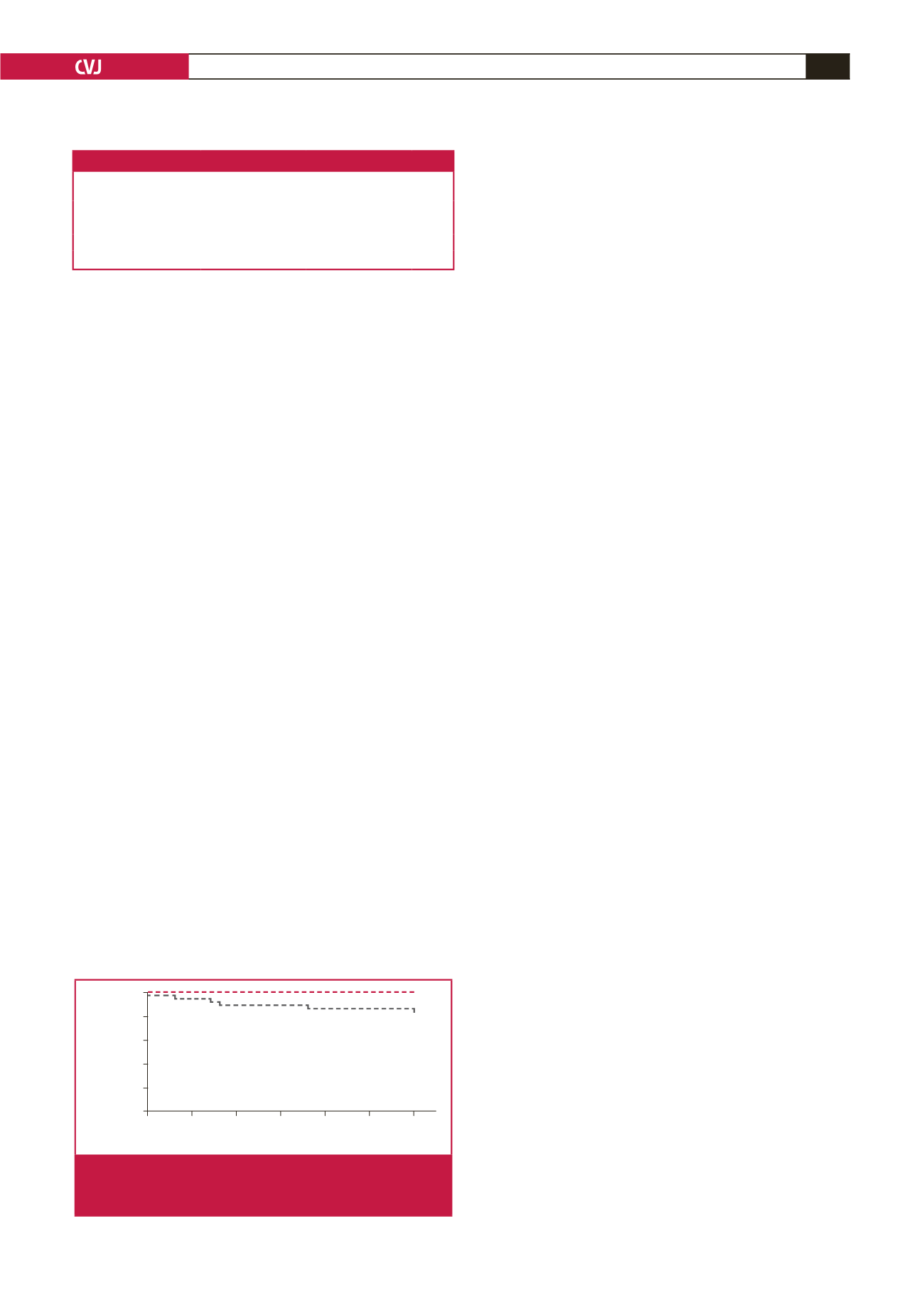
in patients undergoing iloprost infusion therapy. The Kaplan–
Meier analysis revealed a significant difference in survival
between patients with AKI and those without AKI (at 30-day
follow up: 22.2 vs 2%,
p
=
0.001) (Fig. 1).
Discussion
AKI refers to a rapid and reversible decrease in kidney
function that develops within a period of hours or days. In
this retrospective study, we assessed the relationship between
laboratory and clinical parameters and subsequent changes in
kidney function in patients with PAD who developed AKI after
iloprost infusion therapy.
We observed that iloprost infusion therapy led to hypotension
(systolic, diastolic and mean arterial pressure) and a significant
decline in eGFR. Patients who developed AKI were more likely
to have worse renal function at the initiation of therapy than
other patients. In the multivariate analysis, diastolic hypotension,
smoking and lack of ASA treatment were independently
associated with an increased risk of developing AKI. In addition,
AKI was associated with a higher mortality rate at the 30-day
follow up.
In the kidney, prostaglandins uphold the balance between
vasodilation and vasoconstriction to maintain homeostasis and
physiological kidney function.
13,14
Vasodilator prostaglandins
have clinically important side effects that underscore their
potential efficacy in the treatment of severe PAD.
15
In experimental animal studies, iloprost preserved kidney
function against anoxia in rabbits,
16
and had beneficial effects
in I/R-induced renal injury in a rat model.
17
Furthermore, in a
clinical study by Spargias
et al
., iloprost was successfully used
to prevent contrast-mediated nephropathy.
9
However, in these
studies that reported iloprost to be a renoprotective agent, the
selected doses were as low as 1–2 ng/kg/min and the infusion
period lasted approximately four to six hours to avoid systemic
hypotension, and dosing was not repeated.
9,17-19
Hypotension is the principal, dose-dependent side effect of
iloprost. There is evidence that such hypotension is a risk factor
for the development of AKI and it is a commonly encountered
problem in elderly patients withAKI,
20-22
patients with pre-existing
renal insufficiency,
23,24
and patients with low cardiac output states
such as myocardial infarction and congestive cardiac failure.
24-26
We observed that patients who received iloprost had a
significant decrease in systolic, diastolic and mean arterial
pressure compared to baseline, and that relative diastolic
hypotension was a significant risk factor for the development of
AKI. In their study, Liu
et al
. showed an independent association
between the relative decrease in systolic blood pressure and the
development of AKI.
27
Sutton
et al
.
28
used an ischaemic rat
model to demonstrate that the ‘initiation’ phase of AKI, during
which renal blood flow is reduced, is the primary determinant
of GFR.
6
Similarly to these studies, our AKI patients had
significantly lower diastolic blood pressure, causing decreased
renal blood flow and leading to a decline in GFR.
In patients with chronic kidney disease (CKD), the risk of
developing AKI is significantly increased.
29
Co-morbidities such
as diabetes, hypertension and proteinuria in hospitalised patients
were independently associated with an increased risk of AKI,
requiring dialysis.
29,30
Our patients with AKI showed significantly
reduced renal function with significantly higher serum creatinine
levels and lower eGFR at the initiation of iloprost treatment.
These patients were more prone to develop AKI because of the
kidney’s sensitivity to disrupted microperfusion or hypotensive
ischaemia.
Consistent with these findings, smoking and the lack of ASA
use were significant independent predictors for the development
of AKI in our patients. Smoking is a major preventable risk
factor for atherosclerosis. Exposure to cigarette smoke activates a
number of mechanisms predisposing to atherosclerosis, including
thrombosis, vascular inflammation, abnormal vascular growth
and angiogenesis.
31-33
ASA, the fundamental therapy given for PAD, reduces the
risk of cardiovascular events and arterial occlusion. The use of
ASA for primary and secondary prevention of cardiovascular
events in most patients with PAD is supported by excellent
clinical evidence.
34
Based on these data, we can speculate that the
presence of smoking and absence of ASA use were associated
with microvascular ischaemia, which made these patients more
prone to hypotensive AKI.
The mortality rate in AKI patients with CKD was 3.3 times
higher than that of patients without CKD.
35
In our study,
patients with AKI had significantly higher mortality rates over
Table 4. Drug-related side effects
Side effects
Patients with AKI
(
n
=
36)
Patients without AKI
(
n
=
50)
p
-value
Nausea
,
n
(%)
9 (25)
3 (6)
0.172
Flushing,
n
(%)
1 (2.7)
1 (2)
0.660
Headache,
n
(%)
1 (2.7)
3 (6)
0.448
Thrombophylebitis,
n
(%)
0
0
NA
0
50 100 150 200 250 300
Days
Cum survival
1.0
0.8
0.6
0.4
0.2
0.0
No AKI
AKI
+
+
Fig. 1.
The Kaplan–Meier survival analysis between patients
with AKI and those without AKI (at 30-day follow up:
22.2 vs 2%,
p
=
0.001).

















