
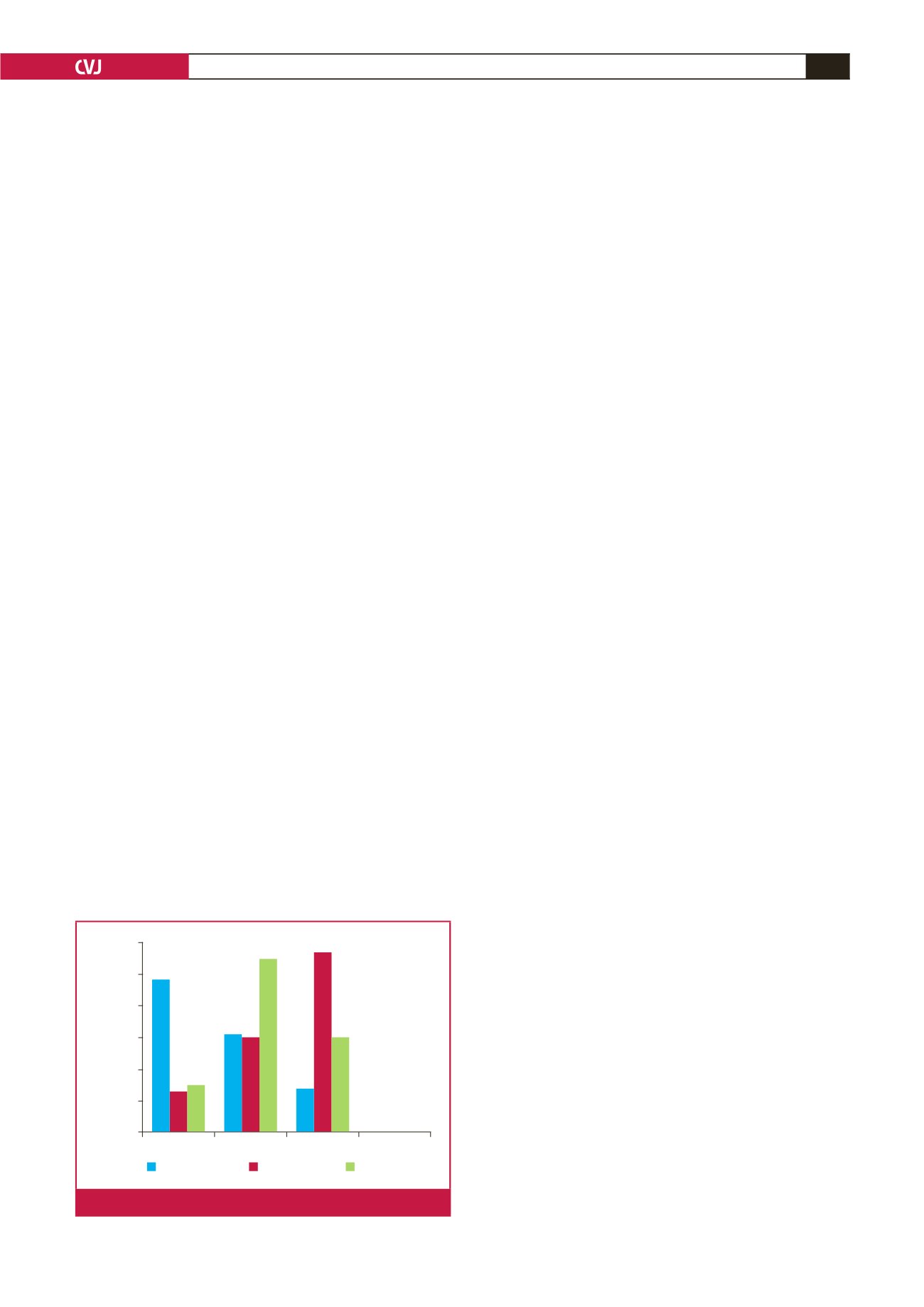
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 29, No 2, March/April 2018
AFRICA
91
thrombo-embolic and atherosclerotic occlusive disease subgroups
are analysed (Table 4).
Multiple publications support the view that patients with
thrombo-embolic ULI, when compared to atherosclerotic
occlusive disease, present at a more advanced age.
1,8
Interestingly,
this finding was not observed in our series. Possible explanations
include the assumed impact that a relatively low life expectancy
(57.7 years for males and 61.4 years for females)
6
may have, as
well as the suspicion of a different risk-factor profile compared
to other research populations.
Both tuberculosis
9
and HIV infection
10
have been identified as
acquired hypercoagulable states. Therefore, with the prevalence
of tuberculosis (25/1000)
11,12
and antenatal HIV infection (33%)
13
in the Western Cape on the rise,
14
it is conceivable that the study
population is at higher risk of developing thrombo-embolic
disease. In the absence of a national registry, a prospective survey
specifically designed to evaluate the impact of tuberculosis and
HIV/AIDS on the incidence and pathogenesis of ULI should be
performed.
Furthermore, all five of the 30-day mortalities were observed
in the embolic acute ULI subgroup. The concept that mortality
following embolectomy is a consequence of the patient’s
co-morbidity rather than the embolus itself, is well supported.
1,15
In our series, post-embolectomy mortality was attributable
to acute coronary syndrome (
n
=
2), acute kidney injury (
n
=
2) and acute respiratory failure (
n
=
1), resulting in a 30-day
all-cause mortality rate of 16.7%. These findings are in keeping
with recent international literature, ranging between eight and
19%.
1,16,17
The only death observed in the chronic ULI group was
as a result of lung carcinoma, documented two years after initial
surgery for atherosclerotic occlusive disease.
Ablative procedures were reported as either primary
(performed at initial procedure) or secondary (following an
attempt at revascularisation), with digital (minor) and above-
or below-elbow (major) amputations separately recorded. The
30-day amputation rate following an attempt at revascularisation
was 12.5%, withmajor (bothprimaryand secondary) amputations
performed in 6.3%.
Patients generally presented late, with 8.6% in the acute
ULI group and 48.3% in the chronic ULI group presenting
with irreversible ischaemia and tissue loss, respectively. When
comparing surgical outcome to that of other case series (see Fig.
2), one has to consider indications for surgery. Units implementing
a more aggressive approach to relatively minor symptoms may
reflect better surgical outcomes, particularly superior limb-salvage
rates. No limbs were amputated in the Deguara
1
series, but the
indications for intervention were not reported.
Of the 64 patients included in this review, seven were
confirmed to be HIV positive by HIV Ag/Ab Combo (ELISA)
testing. However, only 30 patients underwent testing (as indicated
by folder laboratory results sheet or NHLS Disa electronic
results system). One patient developed superficial surgical site
infection and another died of prosthetic graft sepsis, complicated
by an acute bleed. Due to the low rate of HIV testing and small
number of patients involved, it is not possible to reach firm
conclusions regarding clinical outcome in this subgroup of
patients.
Candidates for exclusive endovascular management were
conservatively selected. Five subclavian artery lesions were
managed by primary stent placement, with one lesion stented
after failed percutaneous balloon angioplasty. One patient
sustained a procedure-related complication in the form of
an ipsilateral cerebrovascular incident. All of these patients
attended the six-month follow-up appointment and reported
normal function of the affected upper limb.
Conclusion
Although few firm conclusions could be drawn, this review
has expanded our overall perspective of ULI, specific to the
population we serve. Collaboration between African vascular
units should be encouraged in an attempt to further define the
pattern of ULI by identifying distinct geographical
confounders.
Dr PE Eloff is acknowledged for the initial identification of participants from
a surgical database.
References
1.
Deguara J, Ali T, Modarai B, Burnand KG. Upper limb ischemia: 20
year experience from a single center.
Vasc J
2005;
13
(2): 84–91. DOI:
10.1258/rsmvasc.13.2.84.
2.
Quraishy MS, Cawthorn SJ, Giddings AE. Critical ischaemia of the
upper limb.
J R Soc Med
1992;
85
: 269–273. PMID: 1433088.
3.
Zellweger R, Hess F, Nicol A, Omoshoro-Jones J, Kahn D, Navsaria
P. An analysis of 124 surgically managed brachial artery injuries.
Am J
Surg
2004;
188
: 240–245. DOI: 10.1016/j.amjsurg.2004.02.005.
4.
Gill H, Jenkins W, Edu S, Bekker W, Nicol AJ, Navsaria PH. Civilian
penetrating axillary artery injuries.
World J Surg
2011;
35
: 962–966.
DOI: 10.1007/s00268-011-1008-8.
5.
Sobnach S, Nicol AJ, Nathire H, Edu S, Kahn D, Navsaria PH. An
Analysis of 50 surgically managed penetrating subclavian artery inju-
ries.
Eur J Vasc Endovasc Surg
2010;
39
; 155–159. DOI: 10.1016/j.
ejvs.2009.10.013.
6.
South African Census report 2011.
http://www.statssa.gov.za/publica-tions/P03014/P030142011.pdf.
7.
South African Census report 2001.
https://www.datafirst.uct.ac.za/data-portal/index.php/catalog/96.
8.
Kim S, Kwak H, Chung G, Han Y,
et al
. Acute upper limb ischemia
due to cardiac origin thromboembolism: the usefulness of percutaneous
aspiration Thromboembolectomy via a transbrachial approach.
Korean
J Radiol
2011;
12
(5): 595–601. DOI: 10.3348/kjr.2011.12.5.595.
Tissue necrosis Claudication Rest pain Neuro-vascular
Percentage
60
50
40
30
20
10
0
Current
Roddy
et al
Hughes
et al
Fig. 2.
Summary of chronic ULI presentations.

















