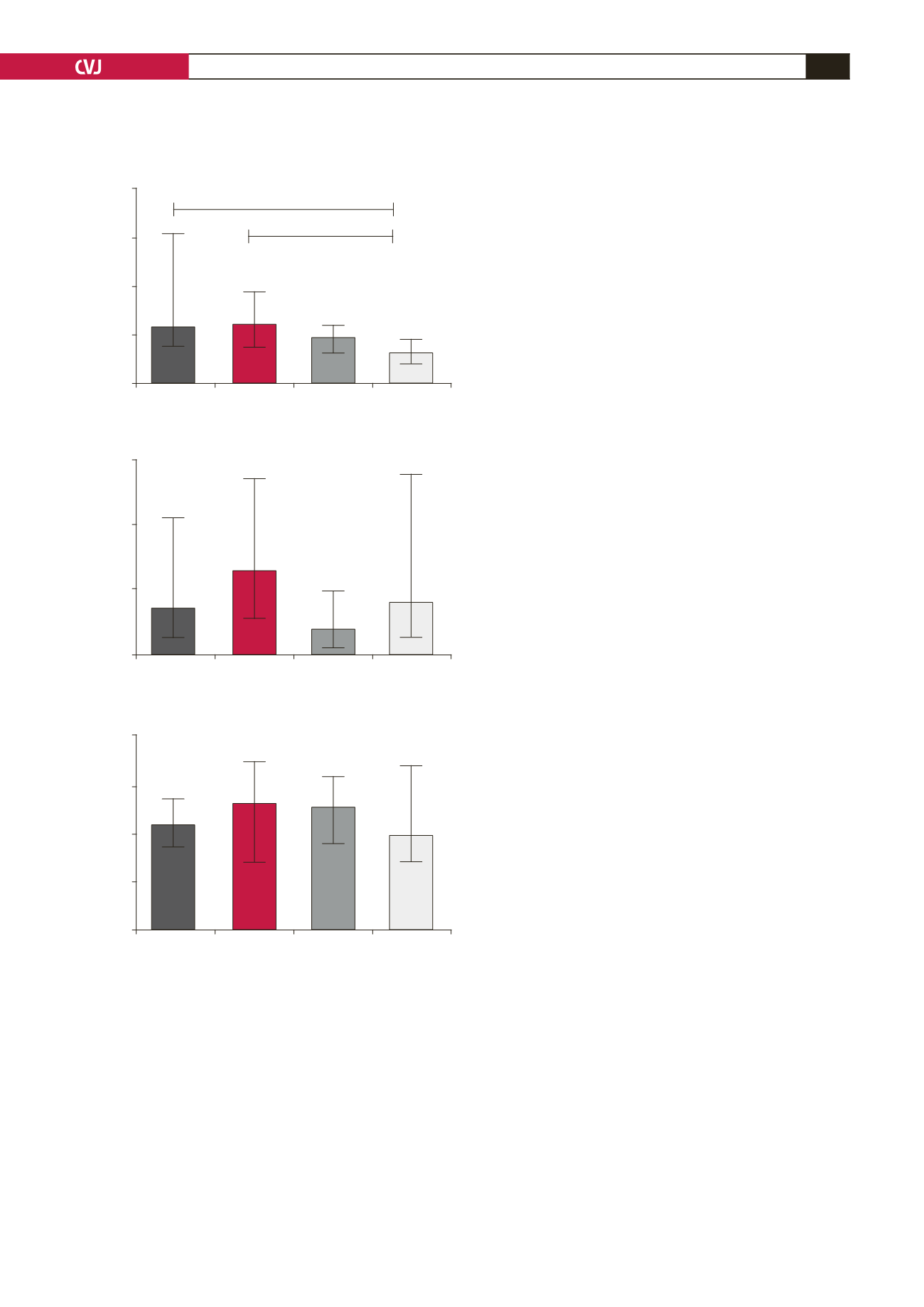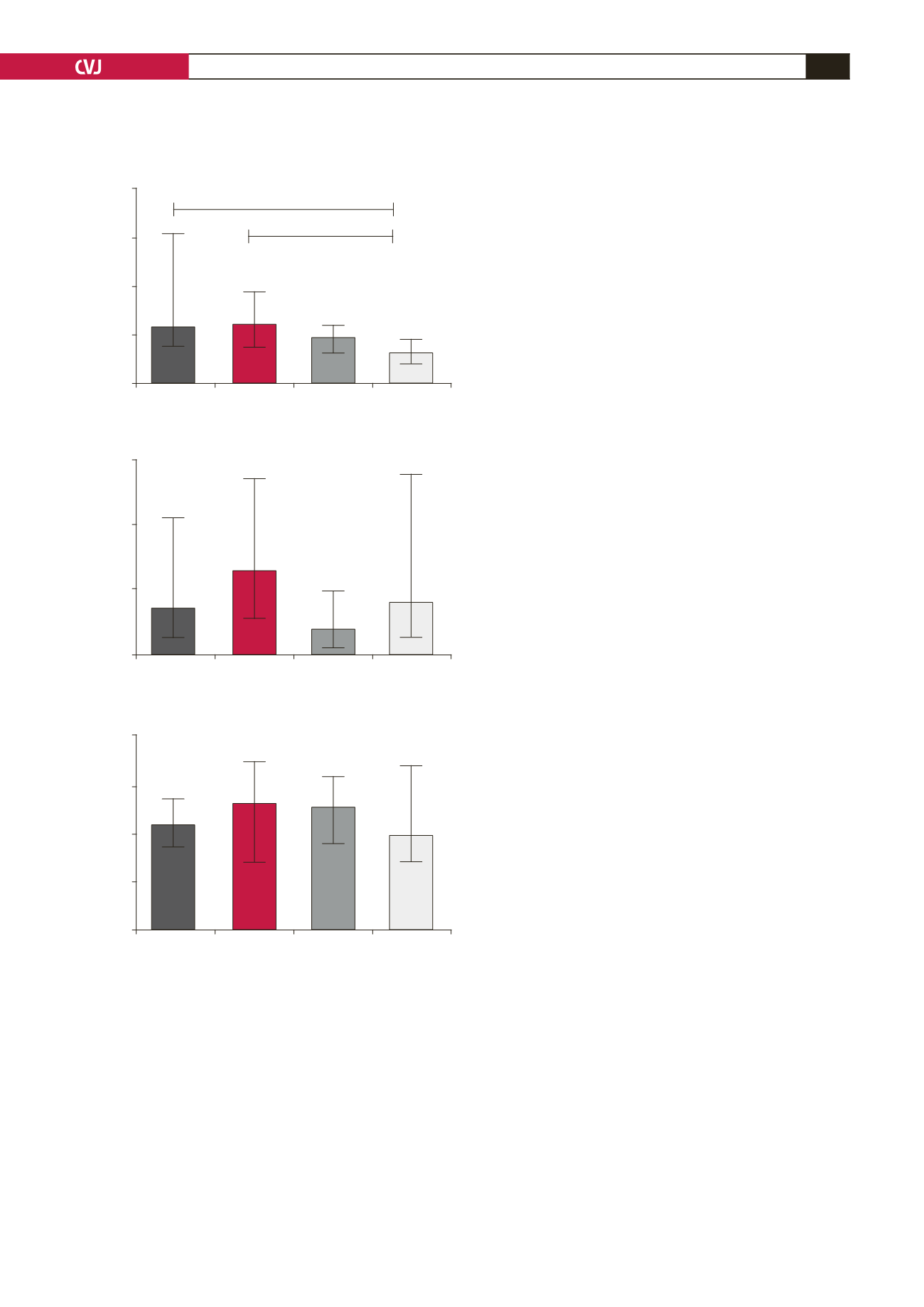
CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 5, June 2013
AFRICA
177
in pre-eclamptics to be lower in view of the fact that the
pre-eclamptics delivered at a lesser gestational age and the fact
that pre-eclampsia is associated with IUGR. Alternatively, this
higher placental weight may be attributed to the late onset of
pre-eclampsia.
Although we did not correlate foetal growth with gestational
age following delivery, there is circumstantial evidence that
women with gestational hypertension and mild pre-eclampsia
tend to have slightly bigger babies and larger placental masses
than their normotensive counterparts at birth. It is plausible that
mild increases in blood pressure could cause a concomitant
increase in placental perfusion pressure and increased oxygen
supply, resulting in increased placental size.
However, a recent epidemiological analysis conducted by
Eskild and Vatten (2010) reported conflicting data with regard
to placental weight.
20
Pre-eclampsia is hypothesised to be due
to placental dysfunction, however these investigators have
suggested that placental weight may not be a risk indicator for
the placental dysfunction evident in pre-eclampsia. In addition,
the placenta is identified as the major angiogenic contributor, and
that the imbalance evident in pre-eclampsia may be associated
with placental hypoxia.
20
Therefore the pre-eclamptic placenta is involved with the
cause of the disease and is implicated in the production of
elevated levels of sFlt1 and sEng.
2,21-23
This elevation is believed
to disrupt the balance of the pro-angiogenic factors, thereby
decreasing their bioavailability, with the subsequent vascular
maladaptation of pre-eclampsia.
Our study further demonstrated variations between the
pro-angiogenic (PlGF and TGF-
β
1
) and anti-angiogenic factors
(sFlt1 and sEng) that occurred in pre-eclamptic (HIV negative
and positive) and normotensive (HIV negative and positive)
pregnancies, lending credence to the anti-angiogenic theory of
pre-eclampsia. To our knowledge, there are no available data that
explore the relationship of HIV with circulating pro-angiogenic
and anti-angiogenic factors in pre-eclampsia.
VEGF is recognised as a powerful endothelial-specific
mitogen and its significant role in angiogenesis is well
documented.
24-26
It is functional through the two high-affinity
receptor tyrosine kinases VEGFR1 (Flt1) and VEGFR2 (Flk1).
PlGF is also a member of the VEGF family, which binds to Flt1,
thereby supplementing the pro-angiogenic effects of VEGF.
24-26
However, a soluble isoform and a splice variant of Flt1 have been
identified as sFlt, which contains a ligand-binding domain but
lacks a trans-membrane and cytoplasmic domain.
27
Karumanchi
and co-workers further demonstrated an excess production of
sFlt1 by the pre-eclamptic placental trophoblasts and subsequent
discharge into the maternal circulation, implicating it as a key
role player in the aetiology of this maternal syndrome.
27
Transforming growth factor-beta (TGF-
β
1
), comprising three
isoforms, is important for the development of the embryo,
inflammation repair, and angiogenesis.
28
TGF-
β
1
,
an isoform
expressed copiously in trophoblasts and endothelial cells,
functions as an apoptotic and proliferative mediator of vascular
endothelial cells, immunosuppression and production of the
cellular matrix.
29
Furthermore, TGF-
β
1
contributes to the normal
placentation through the control of trophoblast invasion.
30
However, in pre-eclampsia it affects trophoblast cell
migration and influences spiral artery conversion by activating
gene transcription and increasing the synthesis of matrix
proteins.
31
It also decreases pericellular proteolysis by decreased
synthesis of proteolytic enzymes such as the serine and matrix
metalloproteinases (MMPs), and increases the synthesis of tissue
inhibitors (TIMPs), thereby modifying the repertoire of cell
adhesion receptors such as the integrins.
31
In the current study we
were unable to demonstrate any significant difference for TGF-
β
1
between the groups.
Endoglin (Eng), a co-receptor for both TGF-
β
1
and TGF-
β
3
,
Fig. 2. Anti-angiogenic ratio of serum concentrations
(medians with interquartile range). (A) sEng/TGF-
β
1,
(B) sFlt1/PlGF and (C) (sFlt1
+
sEng)/PlGF; HIV-positive
pre-eclamptic (P
+
); HIV-negative pre-eclamptic (P–);
HIV-negative normotensive (N–) and HIV-positive normo-
tensive (N
+
).
A
C
B
2.0
1.5
1.0
0.5
0.0
20000
15000
10000
5000
0
150
100
50
0
P+
P–
N–
N+
P+
P–
N–
N+
P+
P–
N–
N+
sEnd/TGF beta 1 (pg/ml)
sFlt-1 + sEng/PIGF (pg/ml)
sFlt-1/PIGF (pg/ml)
*
*