
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 26, No 4, July/August 2015
198
AFRICA
Pre-dilation and pre-stenting was performed in all three cases.
This was achieved using BIB
®
(NuMED, Hopkins, NY, USA)
balloons and bare-metal stents (IntraStent
TM
LD Max
TM
, ev3
Endovascular, Plymouth, USA) in cases 1 and 2. Both patients
needed only one pre-stent to obtain a stable RVOT with no recoil
of stent during balloon deflation and no residual stenosis.
Case 3 was more complicated due to the direct retrosternal
position of the RVOT. The RVOT was severely calcified
and residual stenosis and recoil of a 45-mm covered CP
stent (NuMED, Hopkinton, NY, USA) using a 22-mm BIB
®
warranted a second stent implantation to secure a stable landing
zone. This was achieved using a 36-mm IntraStent
TM
LD Max
TM
(ev3 Endovascular, Plymouth, USA) on a 22-mm BIB
®
. Residual
indentation of the stents was abolished using a high-pressure
balloon (Bard Atlas
®
PTA dilatation catheter, Bard Peripheral
Vascular, Tempe, AZ, USA) (Fig. 3).
Once RVOT rehabilitation was completed, the Melody
®
valves were successfully implanted. Patient 3 needed post-valve
implant dilatation due to the residual stenosis and gradient.
Post-stent implantation gradients were measured using a Multi-
track
TM
angiographic catheter (NuMED, Hopkinton, NY,
USA). At the time of implantation, the decision was made
that the results were satisfactory and no further dilatation was
indicated. Melody
®
valve implantation was successful in all three
patients, with reduction of RVOT gradients and elimination of
the PR (Fig. 5).
Post valve implantation coronary angiograms were
normal with no vessel obstruction. There were no ischaemic
changes on ECG for 48 hours and no pericardial effusion on
echocardiography. The patients were observed overnight and
discharged home within 48 hours.
The patients have now been followed up for two years and
demonstrated RV size and function improvements with normal
pulmonary valve function. Subjectively all patients reported
improved exercise tolerance.
Discussion
Percutaneous pulmonary valve implantation has become an
accepted alternative to surgical pulmonary re-valvulation, with
low morbidity and mortality rates.
4,5
More than 7 000 Melody
®
valves have been implanted in 156 centres worldwide, and these are
the first cases in Africa (direct correspondence with Medtronic).
Indications for percutaneous valve implantation are identical
to surgical indications. Classic indications for Melody
®
valve
implantation include: patients above 20 kg, conduit dysfunction
with stenosis or moderate regurgitation, conduit size
>
16 mm
and
<
22 mm, and favourable RVOT morphology.
4-8
Contra-
indications consist of active endocarditis and a conduit size that
is incompatible with the valve size.
4,7
Helpful information obtained from CTA includes the
anatomical aspects of the RVOT and its spatial relationship to
the coronary arteries. The risk of coronary artery compression
is the most frequent exclusion factor and cause of procedure-
related deaths. Major procedural complications include
dislodgement of the valve, coronary artery compression, rupture
of the homograft, and haemothorax due to pulmonary artery
perforation. Follow-up complications include stent fracture and
endocarditis.
4-6,8,9
Stent fracture rates diminished after the practice
of presenting became common place.
Case 3 demonstrates that percutaneous valve implantation
may be a useful alternative to surgery. The transient chest pain
in this patient was secondary to RVOT stretching and similar to
that of surgery. The benefits of percutaneous valve implantation
include short hospital stay and no ICU care. The availability of
these valves may reduce the duration of RV dysfunction and the
total number of RV–PA conduit replacements.
Conclusion
Introduction of the Melody
®
valve has been proven a safe
and effective alternative to surgery for RVOT re-valvulation.
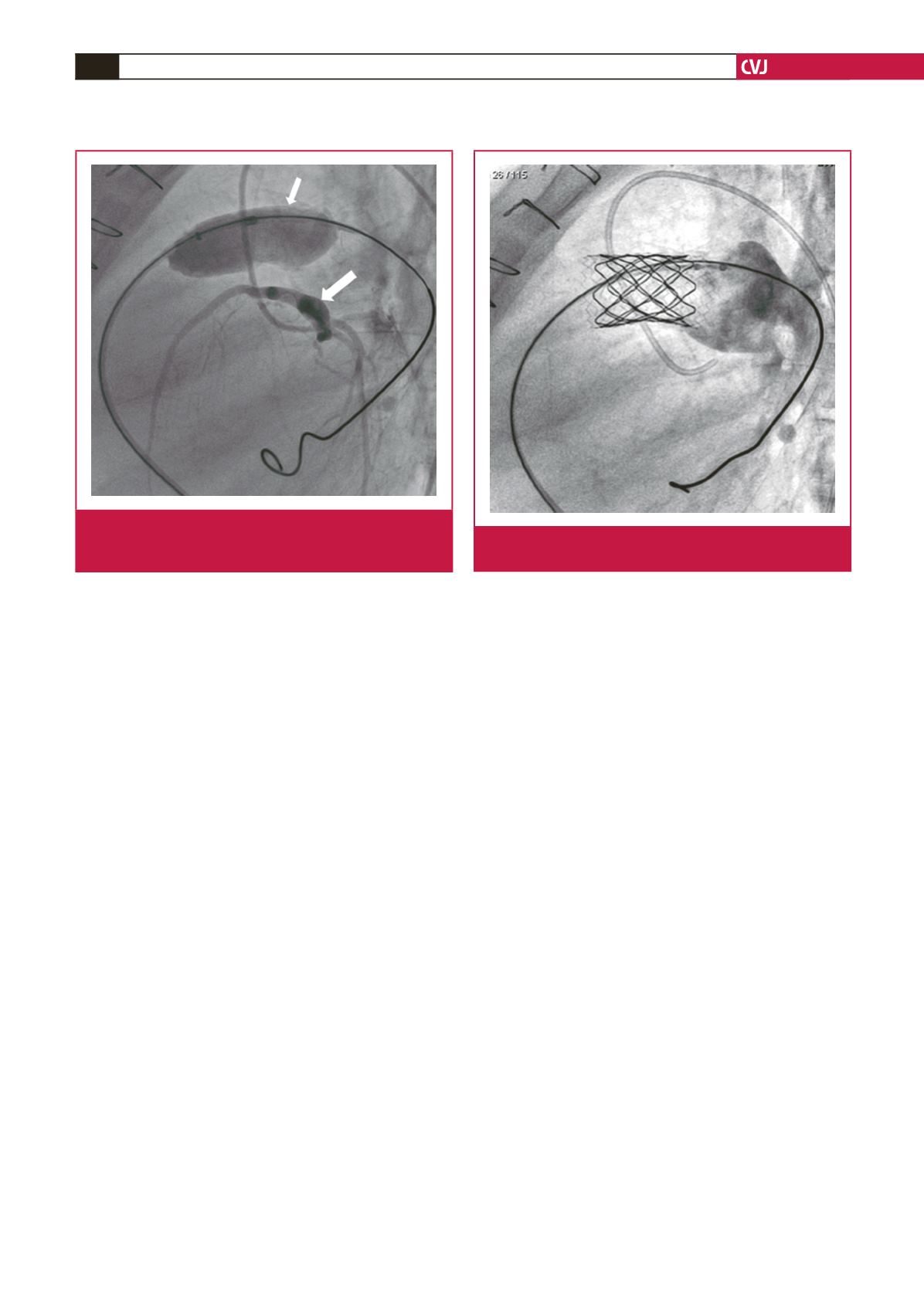
Fig. 4.
Simultaneous inflation of a compliant balloon (small
arrow) in the RVOT and coronary artery angiogram
(large arrow) with no coronary artery obstruction.
Fig. 5.
Melody
®
valve competency was demonstrated using a
Multi-tract
TM
catheter.

















