
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 28, No 5, September/October 2017
AFRICA
307
single cyst with a wall, daughter cysts surrounded by a capsule
with peripheral calcifications, and membrane detachment.
12
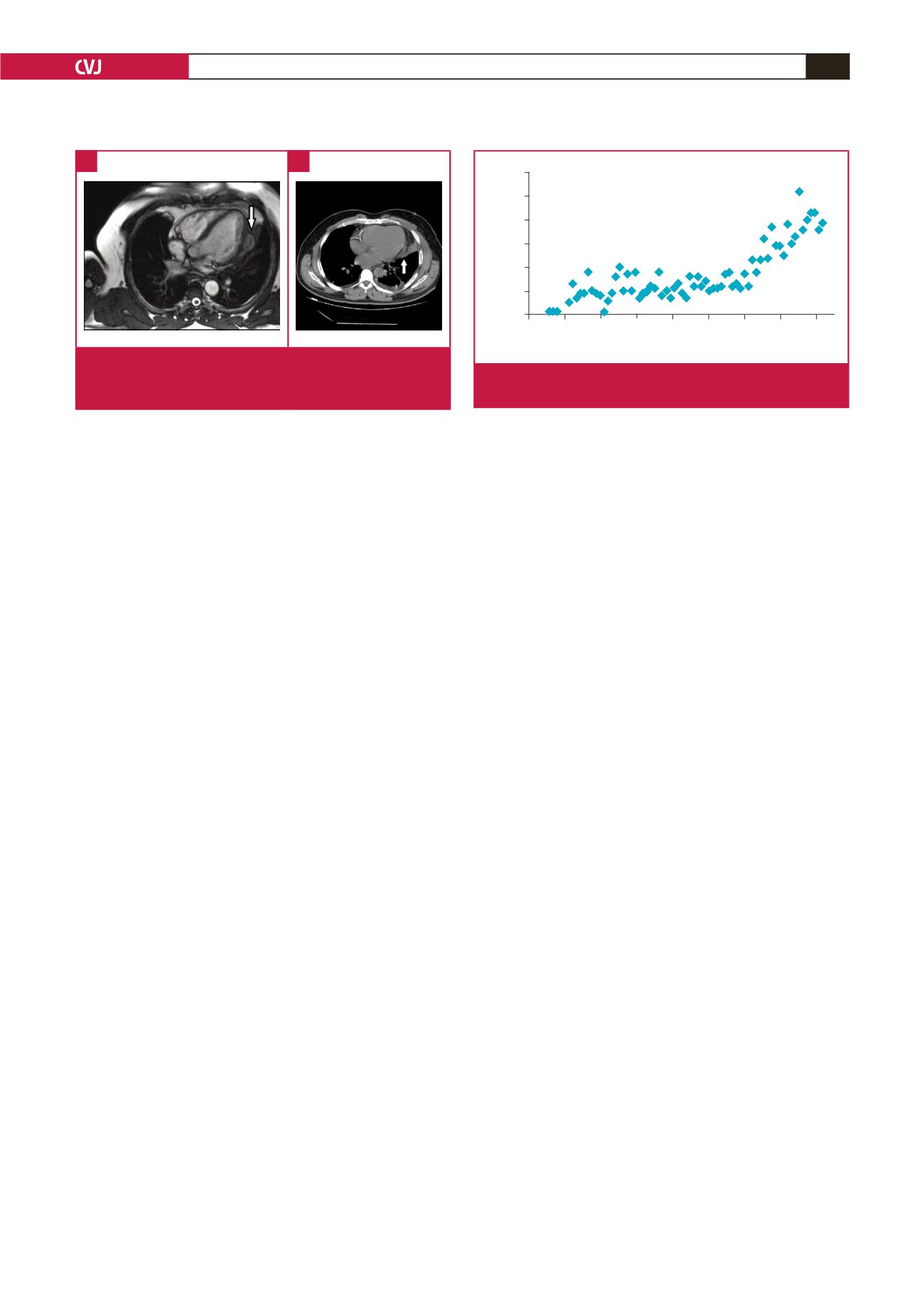
MRI is the most reliable diagnostic modality for CHD; it
depicts the exact anatomical location, and the nature of internal
and external structures. A typical finding on T2-weighted
images is a hypo-intense peripheral ring, representing the peri-
cyst. More specific signs include calcification of the cyst wall,
presence of daughter cysts, and membrane detachment.
CT best shows wall calcification, whereas MRI depicts the
exact anatomical location.
12
In our clinical practice, we use
either CT or MRI prior to surgery to devise a surgical plan
and decide accordingly whether to carry out on- or off-pump
surgery (Fig. 5).
Estimates on the average increase in cyst diameter vary
from about one to 1.5 cm per year.
13
The disease may be silent
for years or cause fatal complications, such as rupture of the
HC, resulting in anaphylaxis. Whatever the location, surgical
removal of the cyst is the definitive treatment for potentially life-
threatening complications, such as rupture, cardiac tamponade
or pulmonary/systemic embolisation.
Most surgeons prefer median sternotomy but on selected and
well-defined lesions, left anterolateral thoracotomy may also be
used. We performed one operation through left anterolateral
thoracotomy in our series. In patients with superficially localised
or pericardial CHD, the off-pump technique can be used,
as in two of our patients. Using the CPB technique may be
mandatory or sometimes beneficial; cross-clamping of the aorta
and pulmonary artery may prevent dissemination of the parasite
to the systemic or pulmonary circulation, thereby preventing
possible pulmonary emboli. After cannulation, our surgical
approach is to puncture and aspirate the cyst contents, sterilise
with 10% saline solution and close the cavity with purse-string
sutures.
After surgery, close follow up of the patients is important to
detect any recurrence or dissemination to other organs. Despite
successful surgery, supplemental medical therapy should be
administered in case of possible cyst rupture and dissemination
of daughter cysts during the operation and to prevent recurrence
of the cysts.
14
We recommend albendazole 400 mg twice a day for
a period of six months.
We searched the literature in the PubMed database using the
words ‘cardiac hydatid cyst’ and the number of reports is shown
in Fig. 6. As can be seen, the number of reports has increased
dramatically over the last decade or two. This may be explained
by more accurate diagnosis using either echocardiographic or
radiological (CT or MRI) studies, and the increased numbers
of open-heart surgery cases since the late 1950s. However, we
should keep in mind that, as people travel more and immigrants
disperse all over the world, an endemic disease will not remain
endemic. Therefore a disease that we thought belonged to the
Old World will also be seen in the New World.
Conclusion
Although CHD is an extremely rare disease, its prevalence
seems to have increased in the last decade. Any patient with
suspected cardiac symptoms suggestive of mass lesions should
be considered for a differential diagnosis of CHD, especially
in developing countries. Definitive treatment is removal of
the cyst combined with medical therapy. Surgery performed
by experienced practitioners provides excellent results when
combined with postoperative medical therapy. We will probably
see more cases, not only in endemic regions, but also in developed
countries in the near future due to the migration of populations.
References
1.
McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis.
Lancet
2003;
362
(9392): 1295–1304.
2.
Rein R, Niggemann B, Runge M. Echinococcosis of the heart.
Herz
1996;
2
1(3): 192–197.
3.
Gormus N, Yeniterzi M, Telli HH, Solak H. The clinical and surgical
features of right-sided intracardiac masses due to echinococcosis.
Heart
Vessels
2004;
19
: 121–124.
4.
Kaplan M, Demirtas M, Cimen S, Ozler A. Cardiac hydatid cysts with
intracavitary expansion.
Ann Thor Surg
2001;
71
: 1587–1590.
5.
Kuruoglu S, Kizilkilic O, Ogut G, Mihmanli I, Akman C, Tanrikulu H.
Primary cardiac hydatid disease: cross-sectional imaging features.
South
Med J
2002;
95
: 1140–1144.
6.
Urbanyi B, Rieckmann C, Hellberg K, Krakau M, Liebau G, Mayer A,
et al
. Myocardial echinococcosis with perforation into the pericardium
.
J Cardiovasc Surg
1991;
32
: 534–538.
7.
Yan F, Huo Q, Abudureheman M, Qiao J, Ma S, Wen H. Surgical treat-
ment and outcomes of cardiac cystic echinococcosis.
Eur J Cardiothorac
Surg
2015;
47
: 1053–1058.
8.
Tuncer E, Turk U, Alioglu E. Cardiac hydatid cyst: An unusual cause of
chest pain.
Int Cardiovasc Res J
2013;
7
(4): 150–151.
9.
Apaydin AZ, Oguz E, Ayik F, Ceylan N. Hydatid cyst confined to the
Year
1940 1950 1960 1970 1980 1990 2000 2010 2020
Number of reports
60
50
40
30
20
10
0
Fig. 6.
Number of cardiac hydatid cyst reports on PubMed
data search.
Fig. 5.
Magnetic resonance (A) and computerised tomogra-
phy (B) images of the cardiac hydatid cyst located on
the left ventricular free wall.
A
B

















