
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 31, No 5, September/October 2020
AFRICA
263
approach to the classification of heart failure emphasises both
the evolution and progression of the disease (Table 1).
This literature review aimed to highlight the concept of
subclinical ATRCD as a stage B heart failure, underline the
importance of its early detection, and emphasise the potential
burden and risk of subclinical ATRCD in the African population.
Our ultimate aim was therefore to draw the attention of African
clinicians in order to improve care of the relevant population.
Concept of subclinical ATRCD
Anthracycline inhibits topoisomerase II (Top2), an essential
enzyme for unwinding deoxyribonucleic acid strands during
deoxyribonucleic acid replication or transcription.
9
High
cumulative use of anthracyclines induces deleterious effects
on cardiomyocytes, endothelial cells, fibroblasts and cardiac
stem cells (Fig. 1). In the cardiac tissue, anthracycline targets
Top2
β
, the primary Top2 isoform in the heart, triggering
profound changes in the transcription, leading to defective
mitochondrial biogenesis and reduced levels of anti-oxidative
enzymes, manifested as increased production of reactive oxygen
species and cardiomyocyte death.
10
Anthracycline has also been shown to reduce coronary
branching, capillary density and the expression of myocardial
vascular growth factors.
11
The number of cardiac progenitor cells
and their ability to differentiate into endothelial cells, smooth
muscle cells or myocytes is also diminished.
11
Therefore the
ability of the heart to adapt to any additional stress is impaired
after exposure to anthracyclines.
Recent study findings suggest that anthracycline cardiotoxicity
represents a continuum that begins with subclinical myocardial
cell injury, followed by an early asymptomatic decline in
LVEF, which can progress to symptomatic heart failure if left
untreated.
12
Not all subclinical LV dysfunctions (stage B heart
failure) will become stage C or D heart failure. However, these
insults enhance cardiac susceptibility to further cardiovascular
stresses (such as pregnancy, surgery, hypertension) or injuries
(radiation, ischaemia) and, ultimately, increase the risk of
premature cardiovascular (CVD) mortality. This phenomenon
has been labelled the multiple-hit hypothesis
13
(Fig. 2).
Cardinale
et al
.
12
suggested that late-onset anthracycline
cardiotoxicity likely reflects the timing of detection, rather than
the timing of the occurrence of cardiotoxicity. These findings,
together with the multiple-hit hypothesis, highlight an urgent
need for the surveillance and management of anthracycline
cardiotoxicity.
Periodic echocardiographic monitoring has been advocated
for this vulnerable population.
2
To further improve early detection
of subclinical LV functional deterioration, guidelines from onco-
cardiologists advise the use of advanced cardiac imaging (global
longitudinal strain, GLS), often combined with the use of
circulating levels of cardiotoxicity biomarkers such as cardiac
troponin.
14
It is therefore recommended to evaluate at baseline
(initiation of anthracycline regimen) LVEF, GLS and circulating
cardiac troponin levels. If any of these three parameters are
abnormal, a cardiology consultation is recommended.
Follow up is recommended at the completion of anthracycline
therapy and six months later for doses
<
240 mg/m
2
or its
equivalent. Once this dose is exceeded, measurements of LVEF,
GLS and troponin level are recommended before each additional
50 mg/m
2
.
2
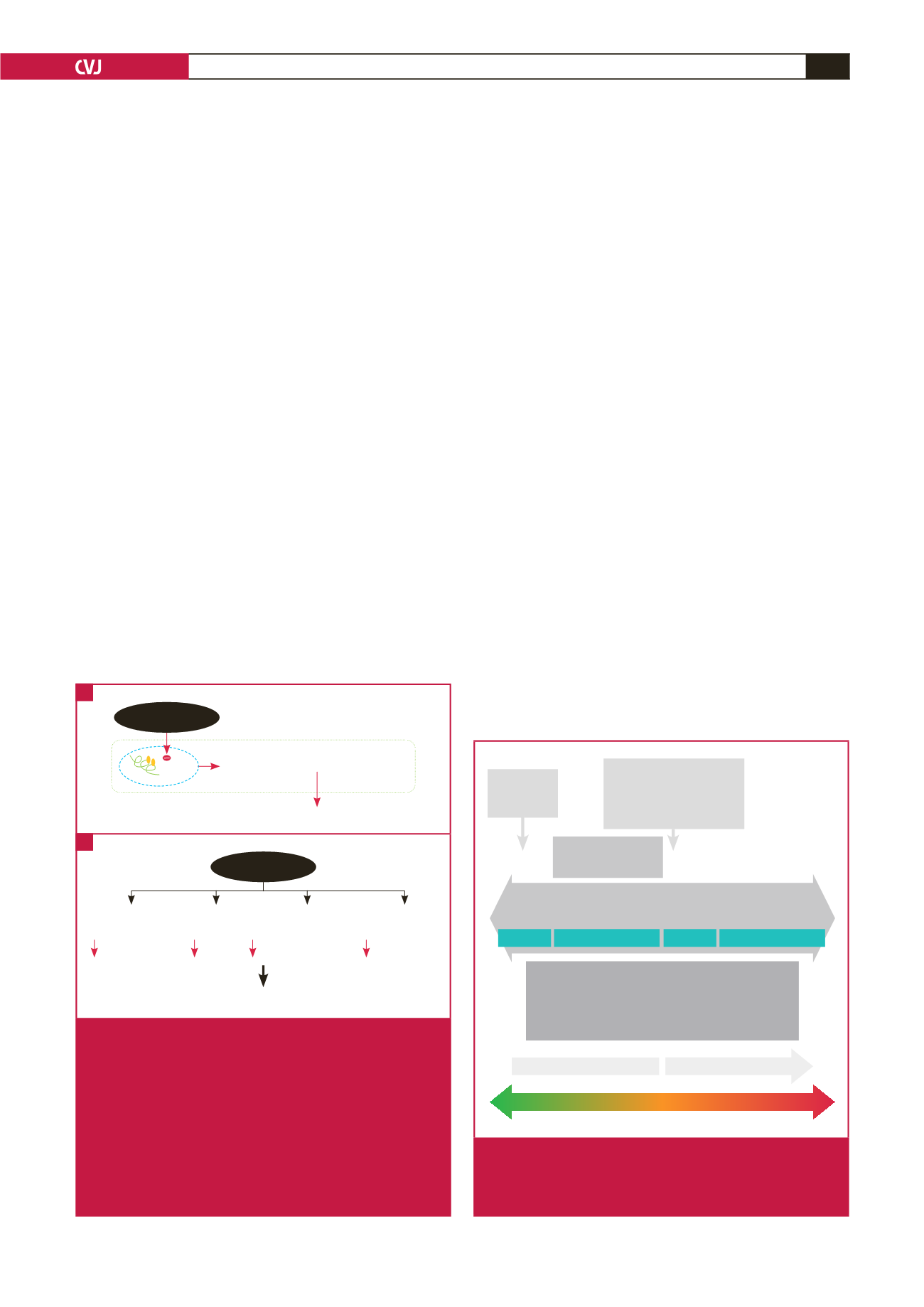
According to recommendations from the American
Reversible
Irreversible
Prognosis
Time from anthracycline therapy
Diagnosis
LVEF assessment
Biomarker (troponin) abnormalities
Myocardial strain imaging (STE)
Symptoms & signs
Initial insult:
anthracycline
therapy
Decreased cardiovas-
cular reserve
Stage A HF
Stage C HF
Stage D HF
Stage B HF
No cardio-
toxicity
Subclinical
myocardial
injury
Asympto-
matic LV
dysfunction
Sympto-
matic HF
Refractory
HF/cardio-
genic shock
Death
Further cardiovascular
stresses (e.g. pregnancy,
surgery, hypertension) or
injury (radiation, ischaemia)
Fig. 2.
Spectrum of ATRCD and the multiple-hit hypothesis.
HF, heart failure; LV, left ventricular; LVEF, left ventricu-
lar ejection fraction; STE, speckle-tracking echocardio-
graphy.
Impaired mitochondrial biogenesis
Cell death
TOP 2
β
DNA
Anthracyclines
Cardiomyocyte Myofibroblasts Endothelial
cells
Progenitor
cells
Contractility
Repair
Neovascularisation Proliferation
Cardiac dysfunction
Anthracyclines
Fig. 1.
Mechanism of anthracycline cardiotoxicity. A: In
cardiac tissue, anthracycline inhibits topoisomerase
II
β
(Top2
β
), triggering profound changes in transcrip-
tion, leading to defective mitochondrial biogenesis,
increased production of reactive oxygen species and
cardiomyocyte death. B: Anthracycline induces delete-
rious effects on cardiomyocytes, endothelial cells,
fibroblasts and cardiac progenitor cells, affects cardiac
contractility and attenuates repair, neovascularisation
and proliferation after injury, thus resulting in cardiac
dysfunction.
B
A



















