CARDIOVASCULAR JOURNAL OF AFRICA • Vol 21, No 3, May/June 2010
160
AFRICA
patient was diuresed and eventually extubated. During his ICU
stay the patient had several runs of non-sustained ventricular
tachycardia.
Given the patient’s ECG, telemetry, echocardiographic and
CT abnormalities as well as evidence of heart failure, there was
a strong suspicion of cardiac sarcoidosis. Cardiac MRI (CMR)
was obtained, which showed severe left ventricular dilatation
(270 ml) and dysfunction (left ventricular ejection fraction of
22%) with dyskinesis of the inferior wall, akinesis of the lateral
wall and septum, and hypokinesis of the apical and anterior
walls.
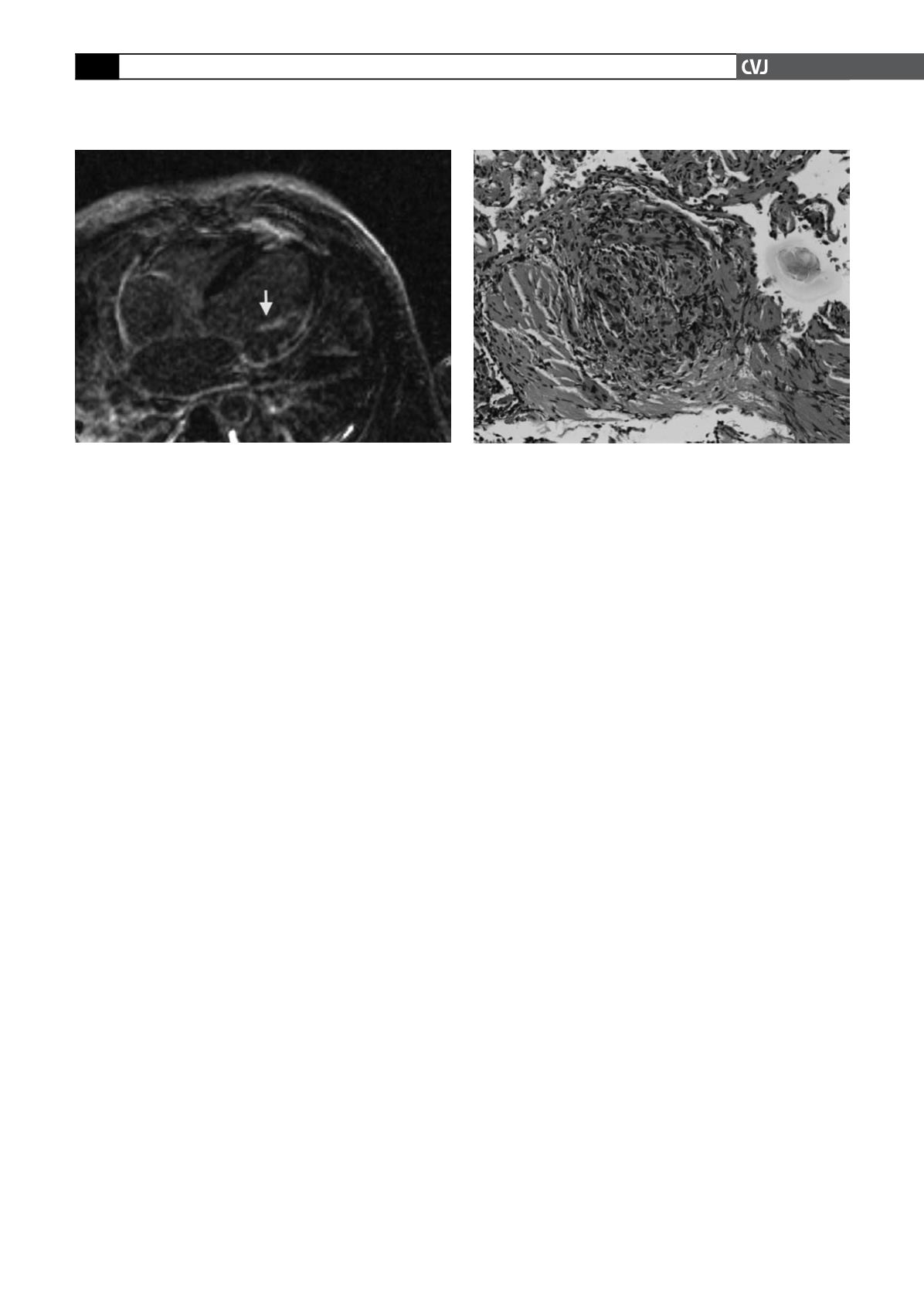
Delayed gadolinium enhancement was seen inferolaterally
(76–100%), laterally (51–75%), septally (26–50%), apically
(26–50%) and anterolaterally (26–50%). There was at least
moderate mitral regurgitation with both anterior and posterior
papillary muscle delayed enhancement (Fig. 3). Delayed gado-
linium enhancement was also seen in the distal right ventricular
free wall and inferior wall. The transmural enhancement in the
inferior and inferolateral walls and subendocardial enhancement
in the distal anterior wall, septum and apex were thought to be
most suggestive of prior infarctions involving the RCA and the
distal LAD.
Right and left heart catheterisations were performed. The
pulmonary pressure was 53/32 mmHg with a mean of 38 mmHg
and a mean wedge pressure of 19 mmHg. Both the left and right
coronary arteries were free of significant obstructive disease.
Endomyocardial biopsy of the right ventricle was obtained,
which showed moderate myofibre hypertrophy and increased
interstitial fibrosis, but no evidence for active inflammation,
myofibre degeneration, vasculitis, granulomas or neoplasia.
Stains for iron and amyloid were negative.
Repeat bronchoscopy was performed with transbronchial
biopsies of the right upper and middle lobes. The pathological
examination revealed lung with mild chronic interstitial inflam-
mation and bronchial wall with mild chronic inflammation and
focal non-necrotising epithelioid granulomas. There was no
evidence of tumour, viral changes or fungal elements (Fig. 4). A
diagnosis of sarcoidosis was made and the patient was started on
prednisone 30 mg per day in addition to his heart failure regimen
(metoprolol succinate 50 mg per day, lisinopril 10 mg per day,
spironolactone 25 mg per day, furosemide 20 mg per day and
aspirin 81 mg per day).
He was doing well one year after diagnosis at clinical follow
up, with subjective improvement in breathing and exercise capac-
ity. His prednisone has been tapered to 30 mg every other day. He
has thus far refused implantation of a cardiodefibrillator.
The patient’s ethnic background, and chest X-ray and chest
CT findings were highly suggestive of sarcoidosis and, as will
be discussed subsequently, this case demonstrates classic cardiac
sarcoidosis presenting with decompensated heart failure as well
as conduction abnormalities secondary to infiltration of the
myocardium and the His-Purkinje system.
Discussion
Cardiac involvement in sarcoidosis is clinically present in 5% of
patients with sarcoidosis, however subclinical involvement based
on autopsy is as high as 20 to 40%.
13-15
Cardiac sarcoidosis can
occur at any time during the clinical course of sarcoidosis, and
can be the presenting feature. The presence of cardiac involve-
ment is associated with a very poor prognosis
8
and accounts for
as many as 13 to 25% of deaths from sarcoidosis.
16
In an autopsy
series, cardiac involvement was seen in 27% of patients whereas
clinical involvement was seen in only 18% (history of heart fail-
ure, arrhythmia or conduction abnormality).
17
Manifestations of cardiac sarcoidosis
Sarcoidosis can affect all tissues and embryological layers of
the heart (Table 1). Granulomatous infiltration can affect the
conduction system, with complete heart block being the most
common presentation in patients with clinical cardiac sarcoido-
sis (23–30%).
6
First-degree atrio-ventricular (AV) block and
bundle branch blocks can also occur.
6,7
Q waves are most likely
indicative of myocardial fibrosis and granulomatous infiltra-
tion but rarely, there can be epicardial coronary artery sarcoid
involvement, which has even presented as an acute coronary
syndrome.
18
Ventricular arrhythmias are due to active granulo-
mas or myocardial fibrosis (healed granulomas) creating foci of
re-entrant activity,
19
and ventricular tachycardia and pre-ventricu-
lar contractions (PVCs) are the second most common presenta-
Fig. 4. Transbronchial biopsy at high power showing a
non-caseating granuloma.
Fig. 3. CMR demonstrating left ventricular wall thinning
and delayed gadolinium enhancement of the papillary
muscle (arrow).


