CARDIOVASCULAR JOURNAL OF AFRICA • Vol 23, No 4, May 2012
226
AFRICA
are also known to rise with increased age.
45
One of the mechanisms contributing to reduced NO levels in
aging may be the increased activity of arginase I.
43,44
Arginase I is
an enzyme that catalyses conversion of L-arginine to L-ornithine
and urea, and it thus competes with eNOS for L-arginine.
43
Hence, the increased activity of this enzyme as observed with
advancing age may result in uncoupling of eNOS, reduced
NO production and hence ED.
43,44
Clearly the balance between
EDRFs and EDCFs is lost with advancing age, establishing
aging as a risk factor for the development of ED. Moreover,
aging is often associated with co-morbid conditions such
as diabetes, hypertension and hypercholesterolaemia, further
exacerbating the risk of developing ED, atherosclerosis and
ultimately cardiovascular diseases.
44
Proposed mechanisms of ED
Oxidative stress appears to be the common underlying cellular
mechanism for the development of ED in all the risk factors
discussed above. According to the literature, cardiovascular
risk factors are associated with upregulation of ROS sources,
especially NADPH oxidase.
7,20
However, other sources of
ROS such as xanthine oxidase, cyclooxygenase (COX) and
mitochondria also play a role.
23
In fact, eNOS
per se
becomes a
potential ROS generator when in the uncoupled state.
20
Harmful
effects of oxidative stress include increasing VSMC proliferation
(resulting in thickening of the vascular wall), endothelial cell
apoptosis, and increased expression and activity of matrix
metalloproteinases, which are involved in the establishment of
an atherosclerotic plaque.
39
Oxidative stress comprises increased rates of oxidant
production and decreased levels of antioxidant activity [e.g.
superoxide dismutase (SOD), vitamin C and E, etc.].
46
Under
physiological conditions, the enzyme SOD regulates the levels
of O
2
–
.
47
However, increased generation of O
2
–
overwhelms the
defensive mechanisms of SOD, leaving O
2
–
free to react with
other molecules, particularly NO, for which it has a greater
affinity.
47
O
2
–
is implicated in the direct induction of ED by the
scavenging of NO, leading to the production of the highly reactive
and harmful reactive nitrogen species (RNS), peroxynitrite.
48
In
fact, the reaction between O
2
–
and NO has been reported to
occur much faster (rate constant
=
6.7
×
10
9
m/s) than that of
dismutation of O
2
–
by SOD (rate constant
=
2.0
×
10
9
m/s).
49
High levels of peroxynitrite are injurious to the cells, oxidatively
damaging DNA, lipids and proteins. In addition to being
cytotoxic, peroxynitrite damages the intricate eNOS structure,
leading to eNOS uncoupling, which further perpetuates the ED
vicious circle
50
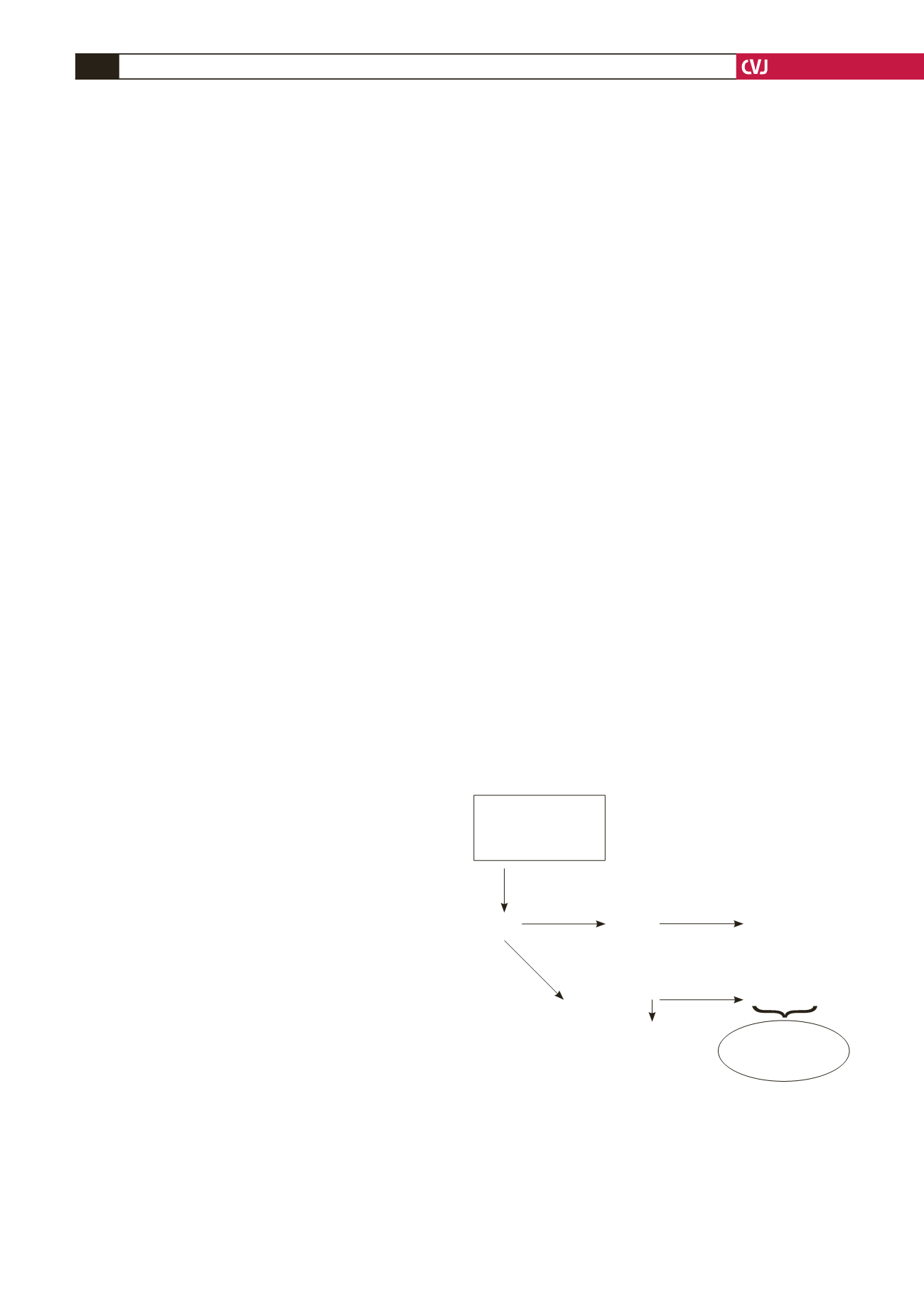
(Fig. 5).
Peroxynitrite has been reported to oxidise the essential
cofactor of eNOS, BH
4
to its inactive form, trihydrobiopterin
radical (BH
3
–
), which in turn leads to uncoupling of eNOS.
20,50,51
Furthermore, peroxynitrite may oxidise the zinc thiolate cluster
in the centre of the eNOS enzyme, resulting in the loss of the
zinc ion and the formation of disulfide bonds between the
enzyme monomers, and thus disruption of the binding site for
BH
4
and L-arginine
20,52
(Fig. 3). Vitamin C is able to recycle
BH
3
–
to BH
4
,
50,51
and supplementation with BH
4
has been
reported to restore endothelial function in conditions such as
insulin resistance, hypercholesterolaemia,
51
diabetes mellitus and
essential hypertension, as well as in chronic smokers.
20
In addition to peroxynitrite-induced eNOS uncoupling, other
oxidants such as hydrogen peroxide have also been shown to
uncouple the enzyme. Therefore, during conditions of oxidative
stress, eNOS deviates from its role of being an essential regulator
of the functioning of the cardiovascular system to being an O
2
–
releasing enzyme. A vicious circle therefore develops, whereby
uncoupled eNOS synthesises O
2
–
at the expense of NO, further
aggravating oxidative stress.
Inflammation is another common underlying mechanism of
ED.
53
Under physiological conditions, the endothelium regulates
vascular inflammation (including expression of adhesion
molecules and leukocyte adhesion) via the release of NO.
54
It is
therefore more likely that ED will promote sustained vascular
inflammation, which is detrimental to the vascular system.
However, several studies have reported that inflammation also
promotes ED and it is therefore recognised as a novel risk factor
for cardiovascular diseases.
53,55
There seems to be a causal relationship between oxidative
stress and inflammation. Oxidative stress may amplify vascular
inflammation signalling pathways,
56
and conversely inflammatory
cells increasingly release O
2
–
. Inflammation is often associated
with the overexpression of inflammatory cytokines such as
tumour necrosis factor-alpha (TNF-
α
) and interleukin-1 (IL-1).
These inflammatory cytokines in turn prompt endothelial cells
or macrophages to express adhesion molecules such as VCAM-
1 and ICAM-1, MCP-1, interleukin-6 (IL-6) resulting in a state
of endothelial activation, which is a precursor of ED
57
(Fig. 1).
The role of TNF-
α
in ED has received considerable attention
in recent years, and is now well appreciated. High levels of
TNF-
α
have been associated with cardiovascular diseases such as
acute myocardial infarction, chronic heart failure, atherosclerosis
and myocarditis.
58
Increased TNF-
α
levels are also significantly
correlated with obesity, which is an independent risk factor for
ED.
59
This inflammatory cytokine has been reported to promote
Fig. 5. Oxidative and nitro-oxidative stress. Superoxide
anion (O
2
–
) released from sources such as NADPH
oxidase, mitochondria and xanthine oxidase is dismutat-
ed to hydrogen peroxide (H
2
O
2
) by superoxide dismutase
(SOD), which is then converted to water and oxygen by
catalase. However, O
2
–
has a higher affinity for NO than
SOD, and when in excess, it preferentially combines with
NO to produce peroxynitrite with various pathophysio-
logical consequences.
Protein nitration
Apoptosis
Necrosis
SOD
K
=
2.0
×
10
9
Catalase
O
2
–
NADPH oxidase
Mitochondria
Xanthine oxidase
=
H
2
O
2
H
2
O
+
O
2
NO
2
–
+
OH
–
NO
=
ONOO
–
eNOS
uncoupling
K
=
6.7
×
10
9


