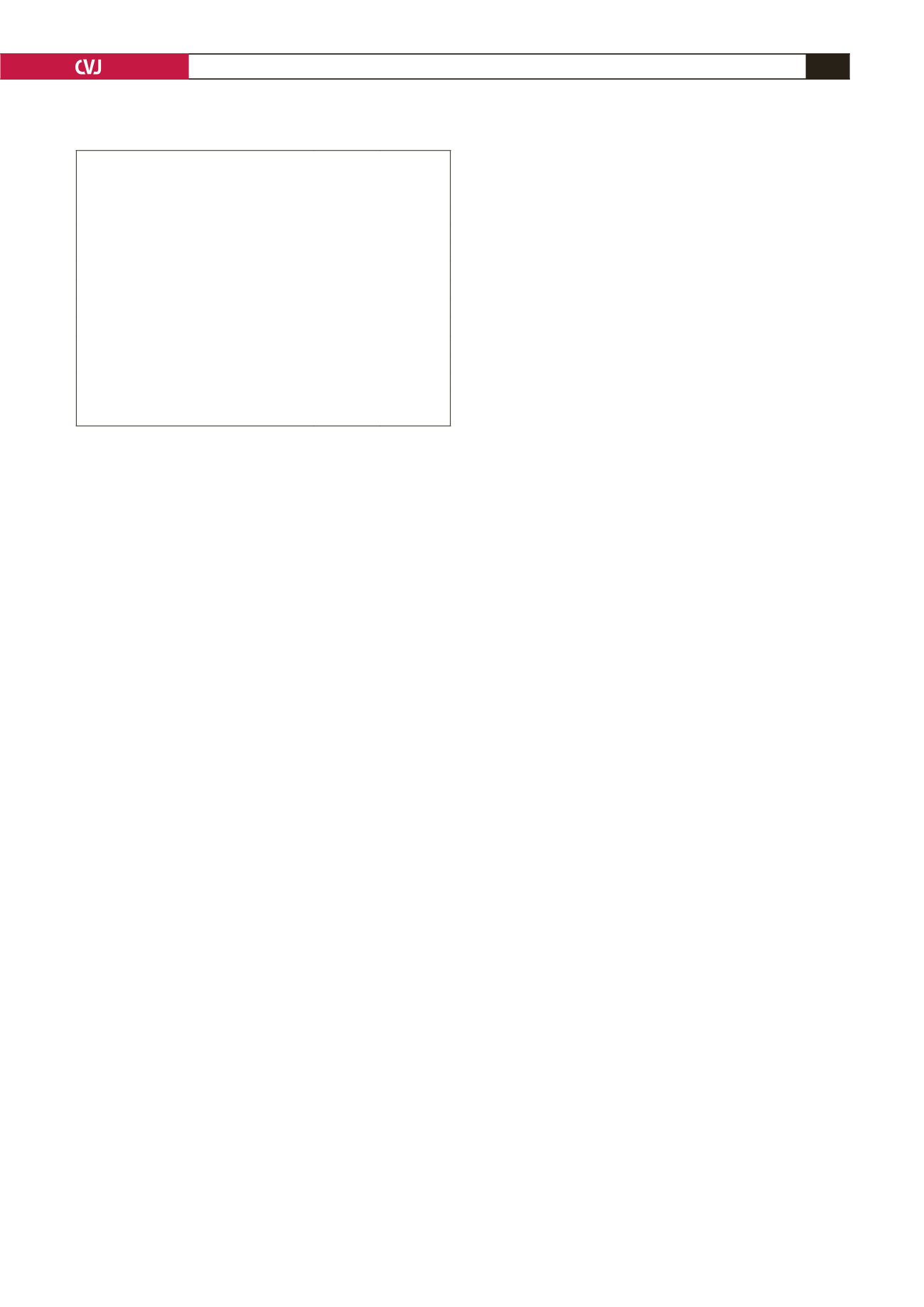
CARDIOVASCULAR JOURNAL OF AFRICA • Vol 23, No 4, May 2012
AFRICA
219
professional should be attached to cardiac clinics to provide and
reinforce appropriate information and prescribe a wide range of
contraceptives where necessary. Similar recommendations have
been made in the Saving Mothers Reports over the last decade
in South Africa.
5
It is difficult to relate the number of pregnancies to the time
from valve replacement. The average age of patients was 24
years, most were primigravidae and the average age of valve
replacement was 12 years.
In our audit, the majority of patients were fitted with MPHV.
The probable reason for this was that MPHV are cheaper and
have greater longevity than bioprosthetic heart valves. However,
because of the propensity of MPHV to undergo thrombosis,
prophylactic anticoagulation is strongly recommended, if not
mandatory.
14-16
Most of our patients with MPHV had either
St Jude or Orynx valves. These new-generation MPHV were
designed to improve blood flow dynamics, prolong longevity and
decrease thrombogenicity. However, our findings and those of
others show that these new-generation MPHV are still associated
with thrombo-embolism.
17-19
There is therefore no doubt that prophylactic anticoagulant
therapy is necessary, but which prophylactic anticoagulation
regimen should be used in young women with MPHV, or should
women requiring heart valve replacement have bioprosthetic
valves inserted prior to pregnancy? Two patients in our study had
bioprosthetic valves. Although patients fitted with these valves
do not require prophylactic anticoagulation, as they are less
thrombogenic than MPHV, the main issues with bioprosthetic
valves are their cost, limited lifespan and the possibility of an
increased risk of structural valve deterioration (SVD) during
pregnancy.
20
In addition, serious SVD can require re-surgery
during pregnancy to replace failing bioprosthetic valves, and
all such operations are associated with mortality and morbidity.
Elkayam and Bitar stated that about 50% of women of child-
bearing age will require valve replacement owing to SVD seven
to 10 years after the original operation.
20
They also reported SVD
in 47% of patients with a history of pregnancy compared with
only 14% in non-pregnant patients.
20
In our study four patients
required replacements; three prior to delivery. All three were
symptomatic, did not have antenatal care and did not respond
to medical treatment. In two of the three cases, the pregnancies
ended in intrauterine deaths, while the baby in the third case
was born alive. In the fourth patient who had a repeat valve
replacement, an elective C/S was planned because she had
severe cephalo-pelvic disproportion and a tight mitral stenosis,
requiring valve replacement. Both mother and baby did well.
These cases illustrate the high perinatal mortality associated with
valve replacements during pregnancy.
The morbidity and mortality associated with anticoagulation
with warfarin is also high and is probably due to high doses
of warfarin in the last two trimesters of pregnancy and the
immediate postpartum period. This is illustrated in our study by
five cases of thrombosis and the maternal death associated with
high doses of warfarin.
There is no current consensus as to the best approach to
anticoagulation in pregnant women with MPHV,
19
as there are no
large randomised studies to guide decision making.
18
In women
with MPHVs, the types of anticoagulation that can be used
during pregnancy include warfarin, UH and LMWH.
Warfarin, a vitamin K antagonist, crosses the placental
barrier, is teratogenic and has been associated with an increased
incidence of spontaneous abortion, prematurity, stillbirths and
central nervous system developmental disorders.
7
Furthermore,
it would appear that the pivotal period for the risk of foetal
congenital abnormalities is between six and 12 weeks of
gestation, resulting in a 6–10% risk of embryopathy.
21,22
There
are also reports suggesting that warfarin is associated with
intracranial foetal bleeding and an increased incidence of
stillbirths.
21,23,24
Nevertheless because of its ease of use and
monitoring, it is commonly prescribed in most under-resourced
countries.
Warfarin is also still used in affluent countries. North
et al
.
reported high foetal waste rates but low valve thrombotic rates in
their series of patients with MPHV using prophylactic warfarin
therapy.
25
If warfarin dosage does not exceed 5 mg daily, the risk
of foetal warfarin embryopathy is extremely small.
7
Vitale
et
al.
studied 58 pregnancies in 43 women with MPHV who took
warfarin
≤
5 mg or
>
5 mg (target INR 2.5–3.5) until delivery.
There were significantly fewer foetal complications in women
taking
≤
5 mg warfarin. It was suggested that warfarin at doses
≤
5 mg to achieve a therapeutic INR may be safe during the first
trimester.
7
In our study, 29 (50%) patients were on warfarin
≤
5 mg in
the first trimester and did not have congenital foetal anomalies,
which was similar to reports from India, Oman and Lebanon.
26,27
However, the four (7%) patients who had embryopathies had
warfarin doses of
>
5 mg daily.
The risk of miscarriages and stillbirths are also reported to be
high in women taking warfarin in the first trimester of pregnancy.
8
In our study, we had 41 live births, with two resulting in neonatal
deaths, 12 miscarriages and six stillbirths. The miscarriages and
stillbirths were probably related to high dosages of warfarin
and lack of close monitoring of anticoagulant therapy.
21,24
This
highlights the need for close and intensive monitoring of
warfarin during pregnancy, particularly at the time of switching
from one type of anticoagulant to another in the first trimester
of pregnancy.
Lack of intensive monitoring at the time of switching from
one form of anticoagulant to another is also demonstrated by
the high wound haematoma rate in our study, namely seven
cases of wound haematomas, three of which required surgical
intervention, and one case of broad ligament haematoma, which
settled on conservative treatment. There were also four cases
TABLE 6. MATERNAL COMPLICATIONS DURINGTHE
ANTEPARTUMAND POSTPARTUM PERIOD
Complications
Number Percentage
Antepartum
Epistaxis 2° anticoagulation
*
3
5
Atrial fibrillation
8
14
Infective endocarditis
6
10
Valve thrombosis
4
7
Warfarin embryopathy
4
7
Postpartum
C/S wound haemotomas
7
12
Primary postpartum haemorrhage
*
1
2
Postpartum haemorrhage 2° anticoagulation
*
3
1
Data expressed as mean (range) or as number (percentage)
*
Patients requiring blood transfusion.