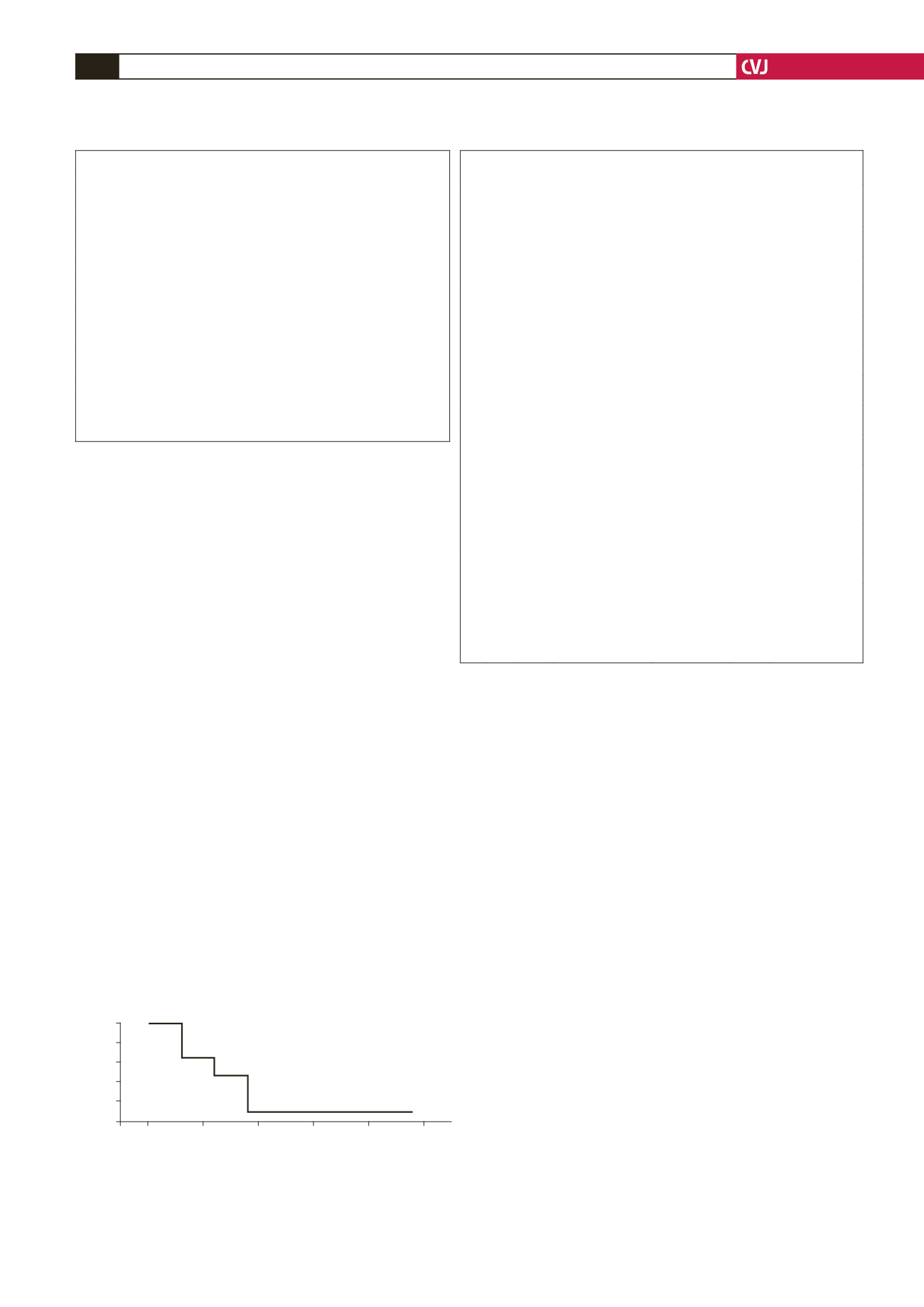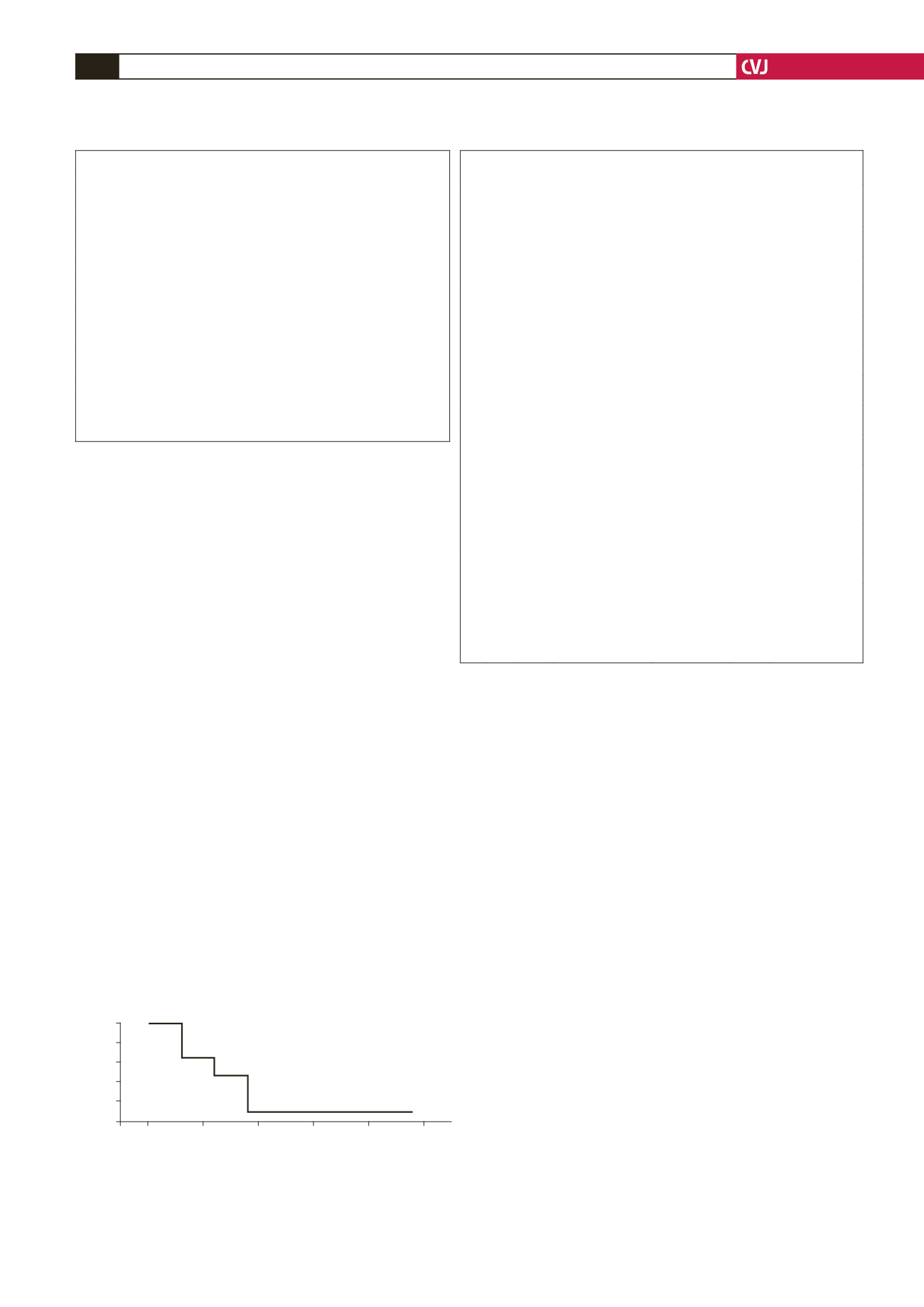
CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 8, September 2013
306
AFRICA
without early mortality. Various complications occurred in
41.6% (
n
=
5) of patients, with more than half the patients having
a prolonged intensive care unit stay.
Total pericardiectomy, which we defined as peeling the
parietal pericardium between the phrenic nerves anteriorly and
peeling its diaphragmatic surface inferiorly, was achieved in
nine patients. Pericardiectomy was limited (partial) in three
patients in whom the decortication border could not satisfactorily
be extended through the lateral aspect of the left ventricle
because of dense calcific adhesions. Also, fragmented areas of
the epicardium had to be left without peeling and could not be
included within the decortication border in five patients.
Although pre-operative ejection fractions of the patients were
within normal ranges, postoperative LCOS was observed in
seven patients. High rates of LCOS may partly have been due
to structural alterations within the ventricle myocardium, which
had developed due to long-lasting constriction during the chronic
disease period. However, inadequate decortication of the left
ventricle was the most probable reason for the development of
high rates of LCOS, but this could not be totally proven.
The initial approach should include a thorough clinical
evaluation, with pericardiocentesis under echocardiographic
guidance, and heart catheterisation available. The existence of
persistent high intracardiac pressures, despite evacuation of the
effusion, is essential for an accurate diagnosis; ECP was present in
only 6.8% of patients undergoing pericardiocentesis in Sagrista-
Sauleda and co-workers’ study.
2
Effective pericardiocentesis is
of therapeutic importance, especially in the setting of pericardial
tamponade.
ECP has increasingly become a subject of intense research.
Hancock was the first to describe the condition as a particular
form of pericardial constriction, which persists despite evacuation
of the compressive pericardial effusion.
1
Although its prevalence
ranged between 2.4 and 14.8%,
3
Cameron reported the proportion
of ECP to be as high as 24% in patients requiring pericardiectomy.
4
The first prospective study by Sagrista-Sauleda and co-workers
identified 15 ECP patients among 190 with tamponade over
a period of 16 years.
2
In this study, an accurate diagnosis of
ECP was made with combined pericardiocentesis and cardiac
catheterisation. The aetiological spectrum was similar to that
reported in previous studies, with a predominance of idiopathic
cases. Other less-frequent causes included post-cardiac surgery,
tuberculosis, post-radiation and neoplasia. Seven of 15 (46%)
patients underwent pericardiectomy within four months after
pericardiocentesis and two patients died in the early postoperative
period.
Patients with cancer were found to have a high mortality and
low pericardiectomy rate, whereas patients with idiopathic causes
of ECP had a low mortality but high pericardiectomy rate. Also,
four of the six survivors ultimately required pericardiectomy.
2
We concluded that the development of persistent constriction is
frequent in ECP and extensive epicardiectomy is the procedure
of choice in patients with persistent heart failure.
A recent systematic review by Ntsekhe
et al
.
3
identified a
pooled prevalence of 4.5% by applying a random-effects model.
The aetiological spectrum was similar to that of previous ECP
and CP series, although neoplastic and traumatic cases were
excluded from the analysis; 26 ECP patients were identified
among 642 subjects derived from five observational studies. The
TABLE 4. OPERATIVEAND POSTOPERATIVE PARAMETERS
Operative parameter
Value (%)
Complete pericardiectomy
9 (75.0)
Time of operation (min)*
90 (90–120)
Ventilation
>
8 hours
4 (33.3)
24 hours bleeding (ml)*
525 (362.5–837.5)
Re-operation for bleeding
2 (16.6)
Fluid removed (ml)*
875 (500–1350)
Transfusion (1 unit of ES)
5 (41.7)
Arrhythmia
3 (25.0)
LCOS
7 (58.3)
ICU stay
>
3 days
7 (58.3)
ICU stay
>
7 days
3 (25)
Peri-operative mortality
0 (0)
ES: erythrocyte suspension; LCOS: low-cardiac output syndrome; ICU: intensive
care unit.
*Data represented as medians with interquartile ranges.
TABLE 5. RESULTS OF PERICARDIAL TISSUE BIOPSYAND FOLLOW-UP
DATA
Pat-
ient
Aetiol-
ogy
Date of
opera-
tion
Intensive care unit
stay
Pericardial
biopsy
Follow
up
(months) Outcome
1 ID 2004 8 days, LCOS, RF,
RDS
Non-specific
inflammation
4.04 Death from
pneumonia + sepsis
2
TB 2004 1 day, uneventful
Granulomatous
inflammation
95.0 NYHA class I
3 MG 2005 7 days, LCOS, RF,
RDS
Neoplastic
involvement
†
2.9 Death from disease
progression
4 MG 2005 2 days, uneventful
Neoplastic
involvement
‡
19.7 Death from disease
progression
5 TB 2006 8 days, re-operation
for bleeding, RF, RD
Granulomatous
inflammation
79.9 NYHA class III
6 ID 2006 6 days, re-operation
for bleeding, RF, RD
Non-specific
inflammation
25.7 Death from
advanced HF
7 MG 2007 2 days, uneventful
Neoplastic
involvement
§
25.6 Death from disease
progression
8 TB 2007 8 days, LCOS, RD Non-specific
inflammation
66.4 NYHA class II
9 ID 2007 1 day, uneventful
Non-specific
inflammation
62.5 NYHA class I
10 ID 2008 5 days, low-dose
inotrope
Non-specific
inflammation
51.3 NYHA class I
11 TB 2008 2 days, uneventful
Granulomatous
inflammation
48.2 NYHA class II
12 ID 2012 3 days, low-dose
inotrope
Non-specific
inflammation
8.9 NYHA class II
ID: idiopathic, TB: tuberculous, MG: malignancy, LCOS: low-cardiac output syndrome,
RF: renal failure, RD: respiratory distress, NYHA: NewYork Heart Association, HF: heart
failure.
†
Neoplastic cell invasion without definitive diagnosis,
‡
Pericardial involvement of malignant mesothelioma (epitolid type),
§
Pericardial involvement of high-grade diffuse B-type cell lymphoma.
Fig. 1. Survival function of the entire group. The figure
displays the survival curve by lifetable analysis. The
overall mortality rate was 41.6%. Cumulative survival was
55.6
±
1.5% at the end of the two-year follow-up period.
1.0
0.9
0.8
0.7
0.6
0.5
0
20
40
60
80
100
Cum survival
n
=
12
Time (months)