
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 26, No 1, January/February 2015
AFRICA
e9
A multidisciplinary decision was made to perform TAVI via
the transfemoral approach. After aortic valve dilatation with a
23-mm balloon, a 26-mm Edwards Sapien XT prosthesis was
successfully implanted.
The patient was asymptomatic post procedurally, however
on her fourth day, control TTE showed prominent left-to-right
systolic shunt at the membranous interventricular septum, with
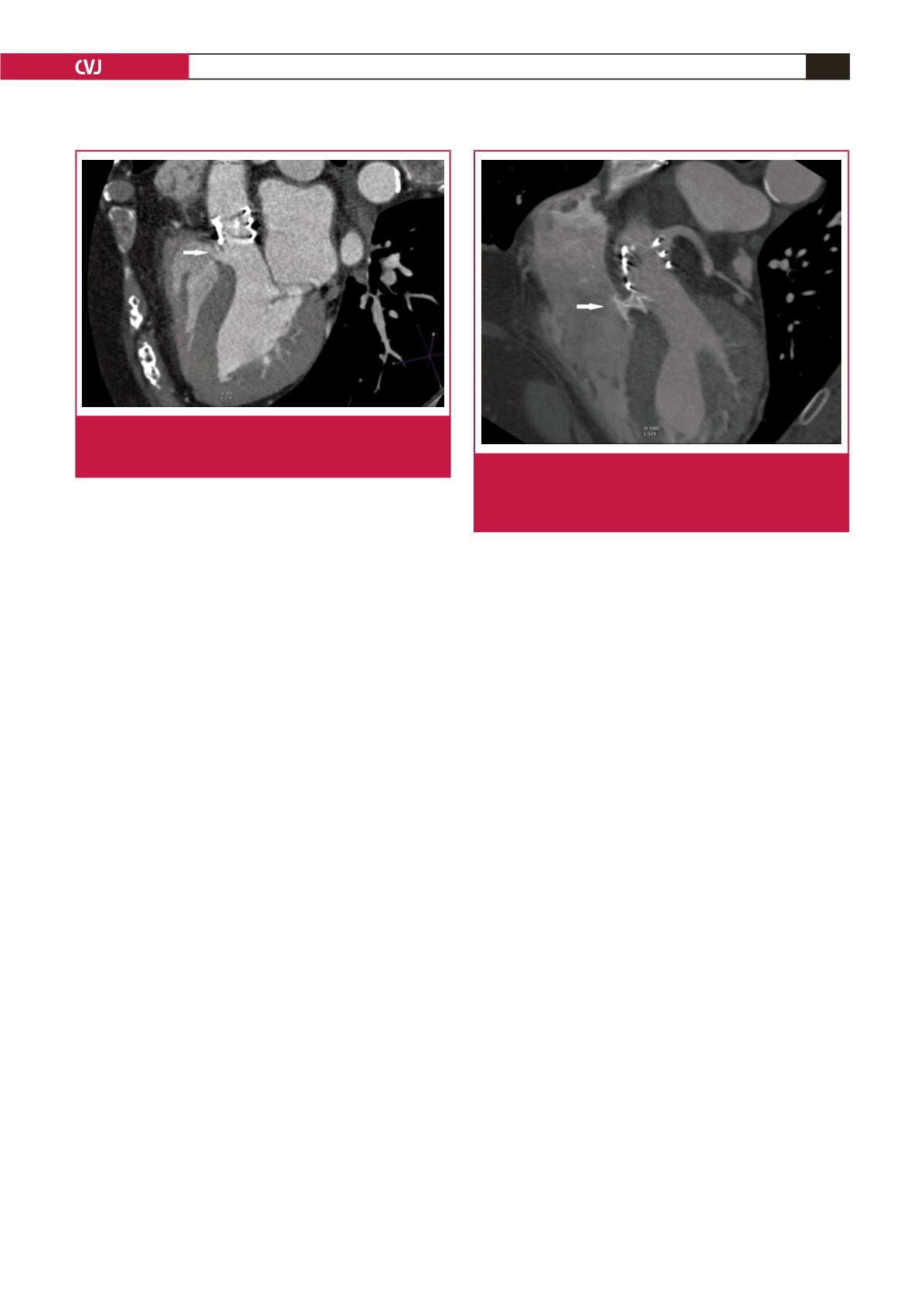
a Qp/Qs ratio of 1.5 (Fig. 1). Multislice CT demonstrated a
free zone of septum about 3–4 mm from the prosthesis and a
membranous type of VSD of 5 mm in diameter, which was not
present before TAVI (Fig. 2).
With these findings, we decided to occlude the defect
percutenously using an 8-mm muscular VSD occluder (AGA
Medical Corp, MN, USA). However, with the close proximity
of the defect to the prosthetic aortic valve, we risked valve
dysfunction. We therefore prepared the cardiovascular team for
emergency implantation of a new prosthetic aortic valve.
Under general anaesthesia the defect was crossed retrogradely
(from prosthetic aortic valve to left ventricle and then right
ventricle), and an arteriovenous loop was obtained. A 7-Fr
TorqVue delivery sheath and dilatator (AGA Medical Corp,
MN, USA) were advanced over the guide wire but we could not
guide the delivery system towards the apex of the left ventricle.
We decided to open the left ventricular disc of the occluder
in the ascending aorta and pull the disc through the prosthetic
valve. Fortunately, the left ventricular disc passed through the
prosthetic valve easily and was placed on the left ventricular
wall. The prosthetic valve was still in the original place, and
aortography showed no aortic regurgitation. Left ventricle
angiography showed the size of the occluder was appropriate
for the defect.
We then opened the right disc of the occluder. Following
the release of the device, left ventricle angiography and
complete transoesophageal echocardiography were performed
and no residual shunting was observed. The aortic prosthesis
was working well. The procedure was finished without any
complications. Control multislice CT showed the VSD occluder
with no residual shunt (Fig. 3).
After three days the patient was discharged home. The patient
was symptom free at the nine-month follow up.
Discussion
VSD is an unusual complication after transfemoral TAVI. In the
literature there are only five reports (one in 2009 and the others
in 2013) of VSD in five patients treated with the Edwards Sapien
XT device and two patients with the CoreValve ReValving
system.
3-7
In most of these cases, post-balloon dilatation of
the device due to aortic regurgitation or oversized prosthesis
implantation have been held responsible for this complication. In
the remaining case there was no explanation other than excessive
aortic calcification.
In our patient there was only mild calcification in the aortic
root with a LVOT size of 23 mm detected by multislice CT
before the procedure. A 26-mm Edwards Sapien XT device was
implanted, and there was no need for post-procedural balloon
dilatation. It was surprising to have a VSD complication in such
a patient.
In our patient, although the defect was haemodynamically
significant, she was asymptomatic, emphasising the importance
of a careful echocardiographic evaluation post procedurally.
Three of the VSD cases after TAVI (two with Edwards Sapien
XT and one with CoreValve) reported in the literature needed no
invasive treatment and were followed up medically. On the other
hand, three patients (all with Edwards Sapien XT) had a surgical
operation for the aortic valve and defect.
4-7
Percutaneous closure
of the VSD after TAVI was reported for only one patient treated
with the CoreValve ReValving system.
3
This procedure has difficulties, such as properly advancing the
delivery sheath into the left ventricle. We overcame this problem
by opening the left ventricular disc in the ascending aorta and
pulling it back through the prosthetic valve. There was, however,
the risk of bioprosthetic aortic valve malposition and acute
aortic regurgitation.
To our knowledge there is no such manoeuvre reported among
the VSD cases with prosthetic aortic valves. We demonstrated
that percutaneous treatment of the VSD after TAVI with an
Edwards Sapien XT prosthesis could be performed without any
complications.
Fig. 2.
Cardiac CT after transcatheter aortic valve implanta-
tion demonstrating ventricular septal defect (white
arrow pointing at the defect).
Fig. 3.
Cardiac CT after occlusion of the ventricular septal
defect (white arrow pointing at the occluder). There is
no interference between the Amplatzer occluder and
Edwards Sapien XT prosthesis.



















