
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 26, No 6, November/December 2015
AFRICA
243
We investigated the effects of Mg
2+
pre-treatment on cardiac
morphological, electrical and haemodynamic changes, and on the
lipid peroxidation profile in a rat model of acute MI induced by ISO.
Methods
Adult male Wistar rats, weighing 250–300 g, were obtained
from the University of Cape Town animal unit and housed in
an air-conditioned animal facility under standard laboratory
conditions (12-hour light/dark cycle, illumination of 323 lux
and temperature of ~22°C). The rats were fed standard rat chow
(Afresh Vention 1, Cape Town, South Africa) and had free access
to food and water.
Experimental procedures were approved by the animal ethics
committee of the Faculty of Health Sciences, University of
Cape Town. All protocols were carried out in compliance with
the
Guide for the Care and Use of Laboratory Animals
[NIH
Publication No. 85 (23), revised 1996].
Animal procedures and experimental protocol
Thirty-five rats were divided into four groups and treated
according to the experimental protocol described below, for
which the timeline is shown in Fig. 1A. Subcutaneous (sc)
injection of ISO at 67 mg/kg in rats is known to produce
histologically detectable MI within 24 hours.
34
In preliminary
tests, we observed that using higher doses of ISO, such as 85 mg/
kg and above, resulted in high mortality rates in our rats.
The ISO-induced MI group (
n
=
9) was pre-treated with
intraperitoneal (ip) injection of physiological saline (2.7 ml/kg)
two hours prior to injection with ISO (67 mg/kg sc), and the ISO
+ Mg
2+
group (
n
=
10) was pre-treated with MgSO
4
(270 mg/kg
ip), which is effective in neuroprotection,
35
two hours prior to
injection with ISO (67 mg/kg sc). The two-hour wait was meant
to avoid possible direct interactions between Mg
2+
and ISO
when co-administered at Mg
2+
peak levels. It was also to allow
adequate time for the onset of any downstream cellular effects of
Mg
2+
treatments that may have occurred prior to the induction
of MI, but before the return of serum Mg
2+
to the baseline levels
expected after 3.5 hours.
35
The Mg
2+
group (
n
=
8) was pre-treated
with MgSO
4
(270 mg/kg ip) two hours prior to saline injection
(3.3 ml/kg sc), and the control group (
n
=
8) was injected with two
drug-equivalent volumes of saline (ip and sc) two hours apart.
Haemodynamic and other
in vivo
measurements were
performed under anaesthesia 24 hours after the treatments.
Rats were anaesthetised with sodium pentobarbitone (60 mg/kg
ip), intubated and mechanically ventilated with room air at 70
strokes/min and 2.5 ml/stroke using a rodent ventilator (Model
681, Harvard Apparatus, Holliston, Massachusetts, USA). The
depth of anaesthesia was adjusted to achieve loss of pedal
withdrawal reflexes, and top-up doses of sodium pentobarbitone
(12 mg/kg ip) were administered where necessary. Rats were
placed on a heating pad (37°C) and the body temperature was
monitored using a rectal probe connected to a T-type pod
transducer (ML312, ADInstruments, Bella Vista, Australia).
Electrocardiogram and haemodynamic recordings
Lead II of a three-lead surface electrocardiogram (ECG) was
used to monitor cardiac electrical changes and compute heart
rate, and was recorded via an animal bio-amplifier (ML136,
ADInstruments, Bella Vista, Australia). Left ventricular blood
pressure was measured with a Millar Mikrotip manometer
(SPC320, Millar, Houston, Texas, USA) inserted through the
right carotid artery in the neck and connected to a bridge
amplifier (ML221, ADInstruments, Bella Vista, Australia). To
prevent drift of pressure from the baseline during recording, the
manometer was cleaned with a physiological detergent (Terg-
A-Zyme, Alconox, New York, USA) and zeroed in water at
37°C. Clot formation around the manometer was prevented by
injecting the rats with heparin (100 IU) intravenously.
After 20 minutes of recordings, the heart was rapidly excised
and retrogradely flushed with cold (4°C) saline through a cannula
inserted into the aorta. The heart was then blotted, weighed and
stored at –20°C for histochemical staining. To prevent damage
of the epicardium due to freeze-drying, the hearts were wrapped
in cling film before being frozen. Pulmonary trunk blood was
collected during heart excision and centrifuged to obtain plasma,
which was snap frozen in liquid nitrogen and stored at –80°C
for lipid peroxidation studies. The other organs such as the liver,
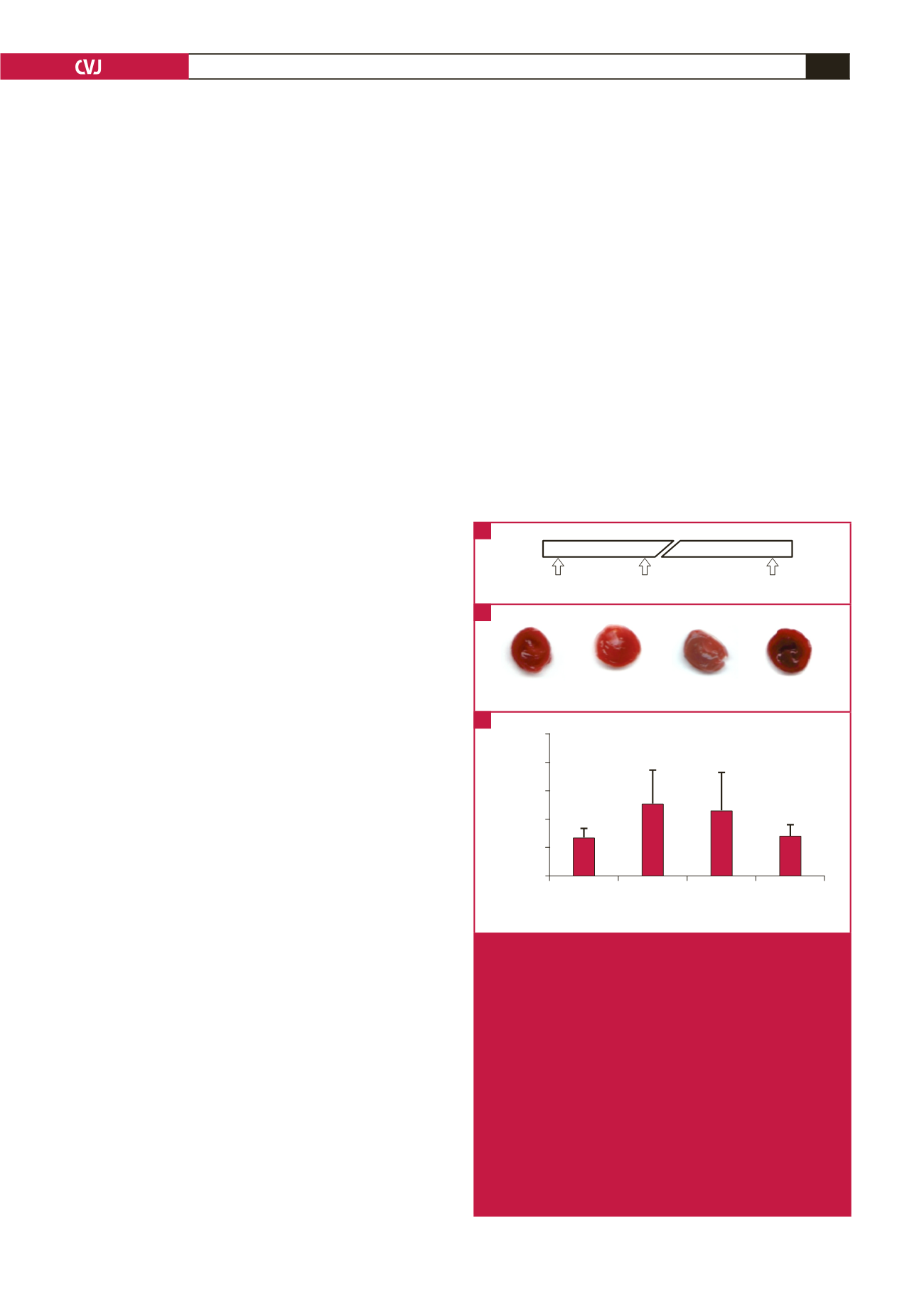
Time 0h
2h
24h
Mg or Saline ISO or Saline
Measurements
Control
ISO ISO + Mg
Mg
Infarct size (%)
Control
ISO ISO + Mg Mg
Treatment
25
20
15
10
5
0
*
*
Fig. 1.
ISO-induced MI and the effects of Mg
2+
pre-treatment.
A: Timeline of experimental protocol. The horizontal bar
represents a 24-hour timeline with a break in the scale.
The times at which rats were treated and at which
in
vivo
and tissue measurements were done are indicated
by arrows. B: Pictures of TTC-stained ventricular slices
cut from four different hearts of rats treated with saline
only (control), ISO and saline (ISO), ISO and Mg
2+
(ISO + Mg), or Mg
2+
and saline (Mg). Viable myocar-
dium stained red (TTC positive), whereas areas of
irreversible infarcts appeared white (TTC negative).
C: Summary data of infarct size in whole ventricles.
The infarct size is expressed as a percentage of the
TTC‑negative area to the total ventricular area. Data
are presented as mean
±
SEM (
n
=
8–10 rats per
group); *
p
<
0.05 (treatment vs control).
A
B
C

















