
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 29, No 4, July/August 2018
250
AFRICA
laboratory, while the other was referred for surgery where the
PDA was ligated and the embolised device was removed.
Discussion
This study shows that transcatheter occlusion of PDAs is safe
and effective in our setting. In particular, the introduction of the
Amplatzer™ devices raised the safety and effectiveness of closure
and a wider spectrum of PDAs can now be closed at the facility.
The need for surgical intervention has declined markedly. Reducing
the number of these cases by using percutaneous closure of PDAs
decreases the time patients with other congenital heart diseases
spend waiting for surgery. The data presented for the period 2008–
2017 showed the predominance of Amplatzer devices continued
and that few complications occurred with these procedures.
The predominance of females in our study is notable and
consistent with what is commonly found in isolated PDAs.
17
Interestingly, in contrast with Krichenko’s description of PDA
shapes, where type B was the second commonest,
1
this shape was
the least common in our population.
The findings of our study add to reports from other settings.
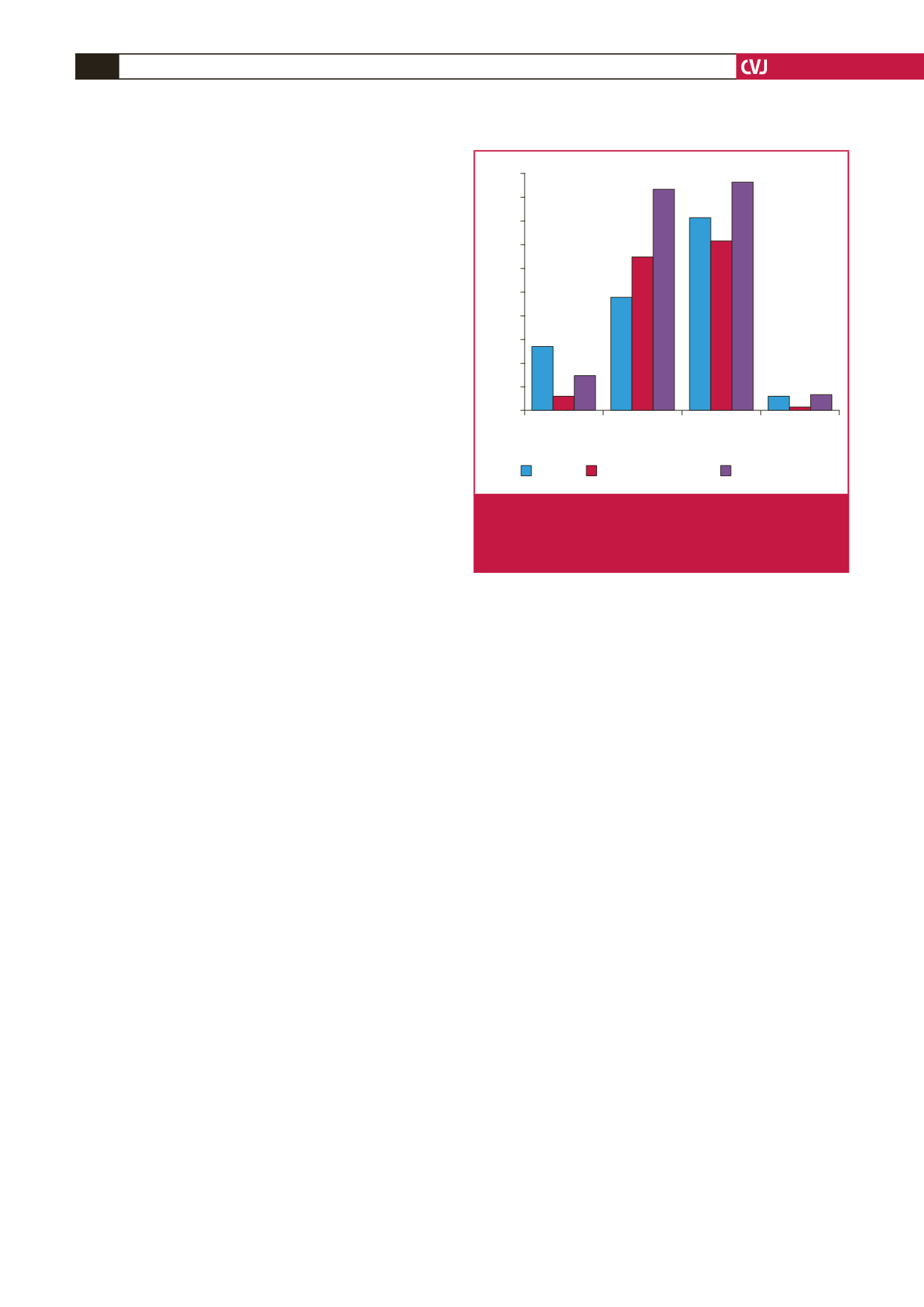
In 2006, Galal
et al
. summarised evidence on PDA occlusion
from 1995 to 2004, covering 21 articles, which included almost
2 800 patients.
6
Our early closure rates are lower than those
reported in that review, although definitions of early closure
differ between our study and the review (Fig. 5). Late closure
rates in our population, however, approximate their reported
range. Late occlusion is perhaps the most important outcome
measure, as it is a key determinant of whether further procedures
are required and of the patient’s risk of endocarditis.
18
The rate
of device embolisation during our study period was acceptable,
falling between the highest and lowest rates reported in the review.
In 2004, Pass published a multicentre review of closure of
PDAs with the Amplatzer™ duct occluder, covering 484 patients
from 25 centres.
19
Our results are very similar to those of the
review, with early closure rates around 90% in the review and in
our study. Total closure rates of 97% in our study were almost
identical to the 98% in that review. The characteristics of our
population and that of the Pass review are similar (e.g. age and
weight), as are the PDA sizes and shunt ratios.
While the above studies were done mostly in high-income
countries, use of these devices has also been assessed in other
middle-income countries.
4,5
A study in Bloemfontein confirmed
the effectiveness of coils in 36 patients in a southern African
environment.
14
Notably, our fluoroscopy time was considerably longer than
all the results reported by Galal
et al
.
6
There are multiple
reasons likely account for this finding. Firstly, we perform a
full diagnostic catheterisation prior to closing the PDA, while
in other settings a briefer catheterisation may have been done,
focused on only closing the duct. Secondly, the introduction of
a new device (Amplatzer) necessitated a considerable learning
curve for the catheterisation team, prolonging the procedure
for the initial patients. The slight increase in number of patients
requiring a repeat procedure to close a PDA around the time that
the Amplatzer devices were introduced was also likely due to the
learning curve.
Finally, our facility is a nationally accredited paediatric
cardiac training centre, with a high turnover of trainees who
each need to perform several procedures in order to become
proficient, and to complete their sub-speciality. As a result, these
procedures most often involve close supervision and teaching of
trainees with little or no experience. The long fluoroscopy time is
an important finding, and results in increased radiation exposure
to both patients and staff. Clearly, attention needs to be paid to
strategies to reduce radiation exposure in our setting.
Study limitations
Types of procedures and outcomes of current care may differ
from that of our study, given that our data collection ended
in 2008 and new devices have been introduced since then.
Nevertheless, these data describe the important transitions in our
unit from one type of device, coils, to the Amplatzer range, and
from surgery to transcatheter closure. Furthermore, this study
is limited in that it is a retrospective record review. While efforts
were made to capture all available data, some information was
missing. Similarly, most patients who underwent surgery prior to
the era of device closure did not undergo cardiac catheterisation
so the data relating to PDA size and shape were not available for
these patients, limiting our ability to compare these and other
patients.
Conclusions
Transcatheter closure of PDAs in the catheterisation laboratory
at Chris Hani Baragwanath Academic Hospital is both safe and
effective. The treatment outcomes are similar to those in high-
income countries, which have considerably more resources for
treating their patient populations. The introduction of a new
device, although associated with a learning curve, broadened the
range of PDAs that could be closed and improved closure rates.
Efforts are needed to address the factors influencing fluoroscopy
times. As more devices become available, the range of PDAs that
could be closed will likely further increase.
CHBH Galal
et al.
– lowest
Galal
et al.
– highest
Fluoroscopy
time (mins)
Early
occlusion (%)
Late
occlusion (%)
Embolisation
(%)
PDA closures
100
90
80
70
60
50
40
30
20
10
0
Fig. 5.
Comparison of PDA closure with coils between our
results and the review reported by Galal
et al
.
6
(lowest
refers to lowest values in studies reviewed, and high-
est refers to highest values in studies reviewed).

















