
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 29, No 4, July/August 2018
AFRICA
253
carbon monoxide may play minor roles. In addition to these
chemical factors, the high pressure in the ductus lumen helps to
keep it open.
After birth, the lowered pulmonary vascular resistance lowers
pressure in the ductus lumen, and PGE
2
decreases both from
loss of placental prostaglandins and a reduced number of PGE
2
receptors in the ductus wall. The increase in arterial oxygen
content has several actions that favour constriction of the
ductus. A membrane-bound cytochrome 450 acts as a transducer
to produce vasoconstrictors.
5
Oxygen inhibits potassium
channels, produces membrane depolarisation, increases smooth
muscle calcium, and induces the formation of endothelin-1; all
these changes stimulate vasoconstriction, although the role of
endothelin-1 is not clear.
Pathophysiology
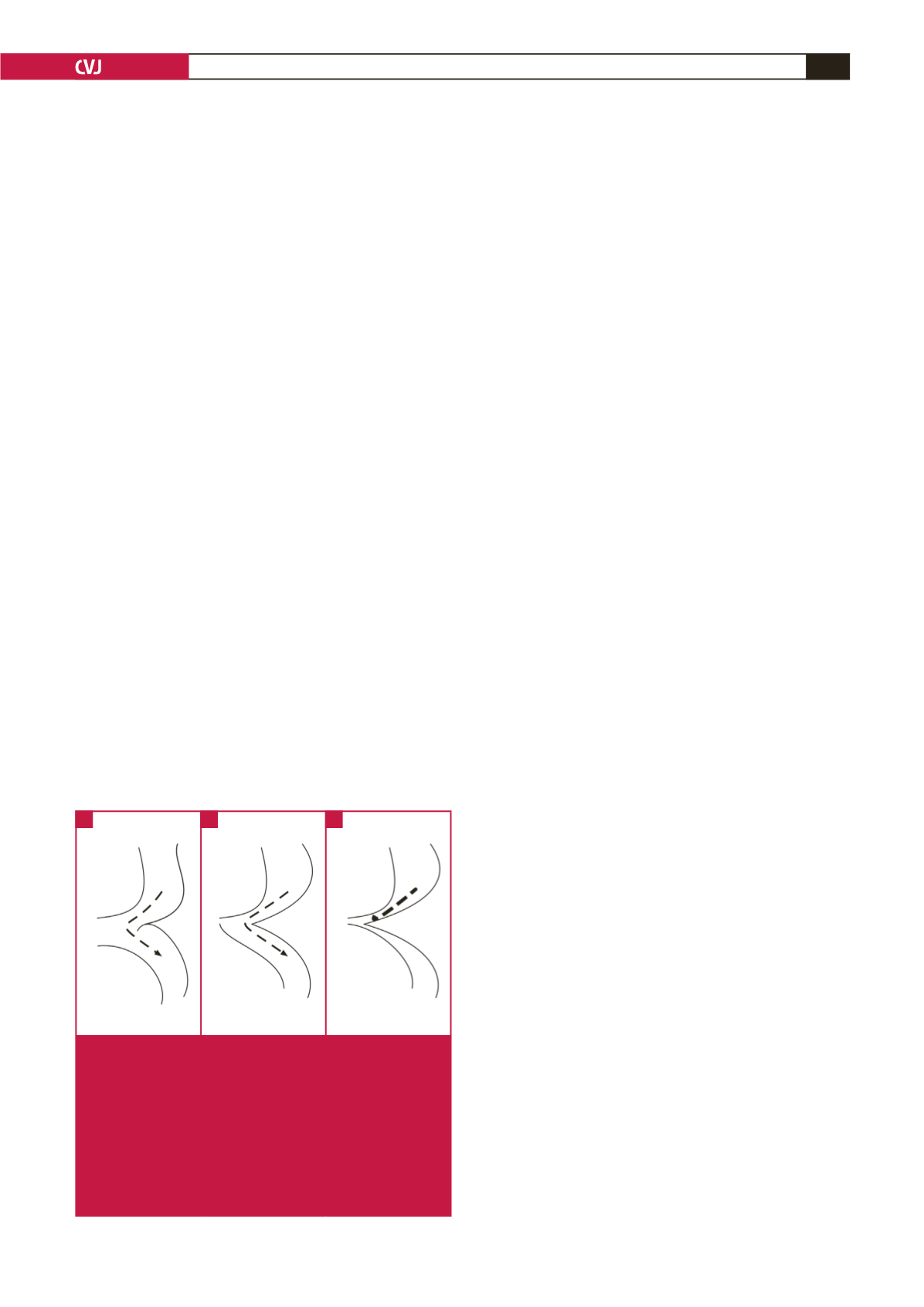
At birth, the ductus is wide open so that despite the shelf, blood
can flow freely from ascending to descending aorta (Fig. 2A).
The ductus closes first at its pulmonary arterial end, but the wide
ductus ampulla provides space for unobstructed flow (Fig. 2B).
This region then narrows further as the ductus ampulla shrinks
and the ductus sling contracts, drawing the lateral wall towards
the closing ampulla (Fig. 2C).
6,7
As a rule of thumb, an artery must be narrowed by more
than 50% before any obstruction occurs, but once this degree
of narrowing occurs, it takes very little additional narrowing
to produce severe obstruction, which can occur very rapidly.
(This functional narrowing can be reversed by infusing PGE
1
.
7,8
)
The sudden severe obstruction overloads the left ventricle,
causing left ventricular failure, pulmonary hypertension, right
ventricular failure and systemic congestion. Pulmonary oedema
is often seen.
If the foramen ovale is patent there will be a left-to-right
atrial shunt that can be large, and if it occurs there may be no
or less pulmonary oedema, because left atrial pressure is lower.
The patient often goes into shock. If the narrowing of the aorta
occurs very slowly, the left ventricle has time to adapt to the
increased pressure load and develop a collateral circulation and
shock and congestive heart failure do not occur. These patients
are usually asymptomatic and are diagnosed at older ages.
Diagnosis: critical coarctation of the aorta in
neonates
Coarctation of the aorta is a treacherous disease that is often
undiagnosed. When neonates present with shock, the most
common cause is sepsis, followed closely by left heart obstruction
(aortic stenosis, coarctation of the aorta), which must always be
excluded. These cardiac lesions usually have insignificant and
non-diagnostic murmurs. The electrocardiograms show right
ventricular hypertrophy, not left ventricular hypertrophy as
expected from a left heart obstructive lesion. A chest radiogram
will show a dilated heart and either pulmonary oedema or a left-
to-right shunt. The liver is usually enlarged.
Recent studies done in Scandinavia found that at least
50% of these neonates were discharged without a diagnosis,
9
and that most were still undiagnosed at five days after birth.
10
In California, Chang
et al
.
11
found that 27% of patients with
coarctation of the aorta died undiagnosed at a median age of
17 days. Ward
et al
.
12
observed that infants with symptomatic
coarctation of the aorta presented between five and 14 days
after birth.
Coarctation can be detected by foetal echocardiography if
performed by an expert, but even then is often not detected.
9
This
is therefore not a diagnostic method for general use.
In older patients the main physical signs are hypertension in
the arms and weak, delayed femoral arterial pulses. These signs
however, are either not detected or are unreliable in neonates
because in most of the infants there is no obstruction to flow
immediately after birth, and hypertension or weak femoral
pulses may take several days to appear. Feeling femoral pulses
may be difficult, especially in plump babies, and by the time
decreased femoral pulses are obvious, the obstruction is fairly
severe. Taking four-limb blood pressures in normal neonates is
not routine, and even if performed, the pressures may not be
accurate. If blood pressures are taken, use the right rather than
the left arm.
When pulse oximetry screening for critical congenital heart
disease in neonates was introduced, several patients with
coarctation of the aorta were detected because they had a right-
to-left shunt through the patent ductus arteriosus. Unfortunately,
this occurs in only a minority of coarctations, perhaps related to
the anatomy of the region, so that pulse oximetry cannot be
relied upon for the diagnosis.
9,13
At present, there is no easy way to make this diagnosis in a
timely fashion. The best way is to have all neonates seen between
three and seven days after birth by a physician or nurse who
can check the femoral pulses. If these are found to be decreased
then immediate transfer to a hospital for treatment should be
done. Waiting is not an option because the likely course is rapid
deterioration. If the patient is already symptomatic, an infusion
of prostaglandin PGE
1
at 0.05 to 0.15 mcg/kg/min will help to
relax the ductus muscle and reduce the obstruction, relieve the
symptoms, allow the left ventricle to recover, and make definitive
treatment safer.
PDA
AA
DA
Coa
PDA
AA
DA
Coa
PDA
AA
DA
Coa
Fig. 2.
A: At birth, the ductus (PDA) is wide open, so
that despite the coarctation shelf (Coa), flow is not
obstructed. Dashed line with arrow shows unimpeded
flow. B: Ductus closes at its connection to the main
pulmonary artery, but its ampulla still provides a
detour for flow. Dashed line with arrow shows unim-
peded flow. C: Narrowing of the ductus ampulla leads
to severe flow obstruction. Heavy dashed line shows
obstructed flow. AA: ascending aorta; DA: descending
aorta.
A
B
C

















