CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 5, June 2013
AFRICA
163
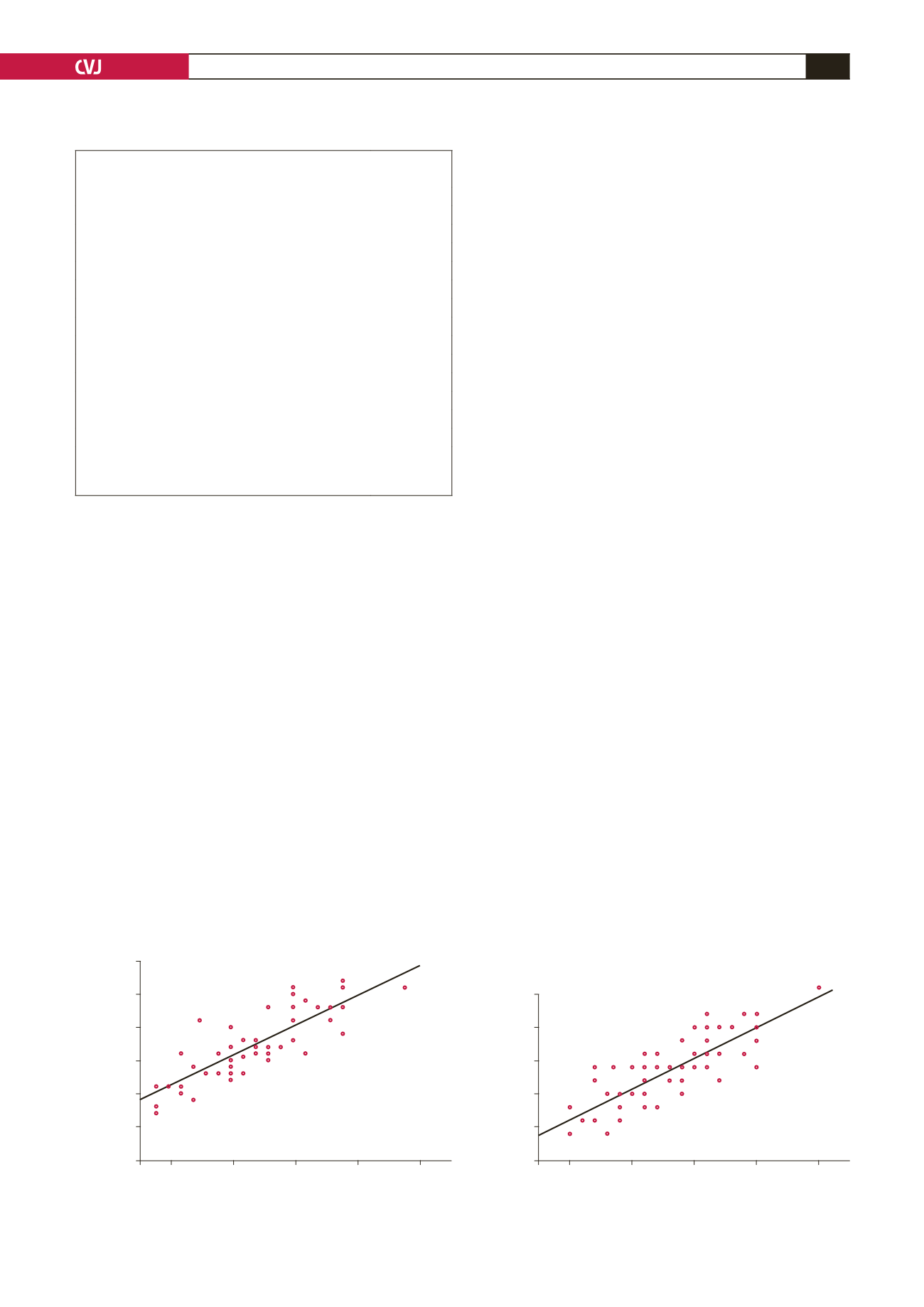
size
+
6.853,
R
=
0.822;
p
<
0.001); Fig. 1 depicts the respective
information.
Discussion
Our results show that the mean TEE-derived size of the ASD was
significantly lower than both the mean diameter of ASD obtained
via balloon sizing and the mean size of the implanted device.
There was good correlation between TEE sizing of the ASD and
the diameter of the deployed device, which makes it feasible to
propose a formula that could be used for the prediction of device
size in ASD occlusion procedures. However it is not sufficient
to predict the exact size of device prior to ASD closure via
echocardiographic evaluation.
In line with our results, some reports have previously indicated
that TEE underestimates ASD size in comparison with the SBD
obtained during catheterisation.
18,28
TEE allows only a limited
view of the ASD morphology. The maximum ASD size might
therefore be underestimated if the probe is not in the same plane
as the largest diameter of the ASD.
18
It could also be attributed
to the intact anatomy of the ASD during echocardiographic
evaluation, in contrast with the disturbed anatomy during device
implantation, which pushes the atrial walls away.
Accordingly, previous studies have revealed a good linear
correlation between TEE-derived ASD size and balloon-sizing
measurements,
10,15,29
and have also evaluated correlations between
TEE measurements and the diameter of the Amplatzer occluder
device. They proposed the following equations to calculate
proper device size: device size
=
2.76
+
1.16
×
TEE defect size,
R
2
=
0.91;
10
device size
=
4.08
+
1.05
×
TEE defect size,
R
=
0.91.
19
The results of the present study confirm these findings, with
some minor differences in the equations. These differences might
be attributed to different sample sizes, technical methods, and
institution standards. Likewise, several other investigators have
confirmed the accuracy of SBD prediction
17
or occluder device
size,
10,19
based on echo measurements.
Some limitations inherent to our study must be taken
into account. We only reviewed the records of 54 patients,
whose procedures were not performed by a single operator.
Consequently, potential differences in the physicians’ experience
levels and in the patient population might have been responsible
for the minor differences between our formula and the formulae
proposed by other investigators.
We excluded multiple ASDs on account of the fact that
accurate assessment of the defect diameter in these situations
was difficult to obtain. The suitability of TEE for the sizing of
multiple defects therefore remains to be elucidated. In addition,
the long-term results of transcatheter ASD closure without
balloon sizing have not been investigated extensively and await
further studies.
Conclusion
Based on this study, a good formula was developed that correlates
TEE measurements of ASD and the size of the implanted device.
However further studies are needed to elucidate whether or not
this formula alone can be used to replace balloon sizing of ASDs.
References
1.
Chen QH, Lu L, Xu XL,Wang Q, Zhao GQ, Hu L,
et al
. Epidemiological
survey of congenital heart disease among people aged from 4 to 18 in
Haidong area of Qinghai province.
Zhonghua yu fang yi xue za zhi
[Chinese J Prevent Med]
2009;
43
: 319.
2.
Feldt RH, Avasthey P, Yoshimasu F, Kurland LT, Titus JL. Incidence
of congenital heart disease in children born to residents of Olmsted
County, Minnesota, 1950–1969.
Mayo Clin Proc
1971;
46
: 794–799.
3.
King TD, Mills NL. Nonoperative closure of atrial septal defects.
TABLE 1. DEMOGRAPHIC, ECHOCARDIOGRAPHICAND
ASD CHARACTERISTICS OF PATIENTS
Cases (
n
=
54)
34.5
±
14.0
Age (years)
12 (22.2)
Male gender
55.6
±
4.4
Ejection fraction (%)
25.6
±
3.8
Left ventricular systolic dimension (mm)
38.4
±
5.3
Left ventricular diastolic dimension (mm)
43.3
±
12.5
Pulmonary artery pressure (mmHg)
2.1
±
0.6
Pulmonary-to-systemic blood flow
38.3
±
5.6
Right ventricular dimension (mm)
25.9
±
8.8
Tricuspid annular plane systolic excursion (TAPSE) (mm)
33.1
±
9.0
Pulmonary artery diameter (mm)
17.8
±
4.5*
+
TEE-derived max defect size (mm)
22.1
±
5.1
+
Balloon occlusive diameter (BOD) (mm)
23.3
±
5.1
Device size (mm)
Data are presented as mean
±
SD or
n
(%). TEE: transoesophageal echo-
cardiography; *
p
< 0.001 compared to the BOD;
+
p
<
0.001 compared to
the device size.
Fig. 1. The relationship between final device size and maximal ASD diameter measured via (A) TEE, (B) balloon sizing.
35
30
25
20
15
10
10
15
20
25
30
Ballon occlusive diameter (mm)
TEE-derived max ASD size (mm)
Y
=
6.212
+
0.898
X
R
=
0.824
p
-value
<
0.001
35
30
25
20
15
10
15
20
25
30
Deployed device size (mm)
TEE-derived max ASD size (mm)
Y
=
6.853
+
0.928
X
R
=
0.822
p
-value
<
0.001
A
B


