
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 28, No 4, July/August 2017
AFRICA
213
These findings conform to features described in previous
works.
4,6,23
Right ventricular EMF echocardiographic features
included fibrosis of the apex, right ventricular free wall and
anterior interventricular septum with obliteration of the
ventricular cavity and dilated right atrium (Fig. 3A). The
pericardium was also found to be affected and looked fibrosed
and thickened, with mild-to-moderate effusion in the majority
of cases (87%). Severe effusion (32 mm) was noted in one case,
without evidence of cardiac tamponade, possibly due to the
chronicity of the disease.
As a result of fibrosis, the three layers forming the heart wall
were identified as separate layers, especially in the posterior
ventricular wall. This feature resulted in a form of ‘layering’
and is shown in Fig. 3 in both the short-axis (3A) and M-mode
views (3B). Layering can be seen on all left ventricular walls,
specifically the lateral and posterior walls. Although the thick
fibrosis of the three separate layers was recognised in post
mortem findings reported by Davies,
24
echocardiographic images
of layering have not been reported before.
New echocardiographic features
Endocardial fibrous shelf:
in countries where EMF is prevalent,
the rates of rheumatic heart disease are also high and diagnostic
difficulties arise in differentiating patients with mitral stenosis
from those with EMF. A new echocardiographic feature, the
endocardial fibrous shelf (EFS) seen in Fig. 5A–D provides
useful diagnostic help.
These echocardiographic images correlate well with a
previously recognised pathological finding first described by
Davies in 1955. He reported that the posterior mitral cusp was
completely immobilised by adherence to the endocardium of the
posterior wall of the ventricle, and the end result was a fibrous
surface running straight down from the atrium to the ventricle
where the cusp had become embedded.
22
Davies further added
that in other cases, the remains of the cusp projected as a short,
thick shelf. This finding is shown echocardiographically by an
immobile posterior mitral leaflet tethered to the endocardium
and appearing like a solid shelf. The anterior mitral valve leaflet,
although moderately thick, moves freely, while the whole mitral
structure becomes reduced to a single leaflet valve.
The echocardiographic endocardial fibrous shelf can be
visualised in the modified APLX in all cases of left ventricular
and biventricular EMF and provides a mark for differentiation
between EMF of the left ventricle and rheumatic mitral stenosis,
where the leaflets and subvalvular structure are fibrosed and may
be calcified but the posterior LV wall remains free.
Endomyocardiopericardial fibrosis: in three cases of advanced
EMF, a dense fibrous pericardium and pericardial calcification
were seen (Fig. 6). This entity behaved clinically like constrictive
pericarditis, as the three patients presented with tachycardia,
ascites and gross oedema of the ankles. We opted to give this type
of EMF, inwhich the pericardiumplayed a significant pathological
and clinical role, the name endomyocardiopericardial fibrosis
(EMPF), and considered it a cause of pericardial constriction.
Although EMF is among the common causes of restrictive
cardiomyopathy, its role in pericardial constriction has not been
described before. Despite the fact that an endomyocardial biopsy
from patients with both tuberculous constrictive pericarditis
and endomyocardial fibrosis revealed similar histopathological
changes of endocardial thickening and focal myofibrosis,
evidence to support pericardial constriction in EMF could not
be confirmed.
25
The echocardiographic and clinical presentation
of patients with EMPF lends support to pericardial constriction
in association with EMF.
Thedifferentiationof EMFfromhypertrophiccardiomyopathy
(HCM), especially the apical type, can be difficult. However our
observations are consistent with the view of Fawzy, Ziady and
Halilm in that with EMF, apical obliteration appears during
both systole and diastole, in contrast to HCM where it occurs
only in systole.
26
One additional observation is the characteristically huge left
atrium (91 mm in one case), which could not be seen, even in
cases of severe mitral stenosis. Among the explanations offered
were the obliteration of the ventricular cavity, and hence the
increase in filling pressure, together with the additional volume
load due to mitral regurgitation.
This study has provided high-definition images of the
main diagnostic features of EMF. Images of layering provide
additional identification of this multi-layer disease. This study
has described and shown images of a new echocardiographic
feature: the endocardial fibrous shelf, which offers an additional
feature for left ventricular EMF. We also report a new entity,
EMPF, a form of advanced EMF that clinically behaved like
constrictive pericarditis.
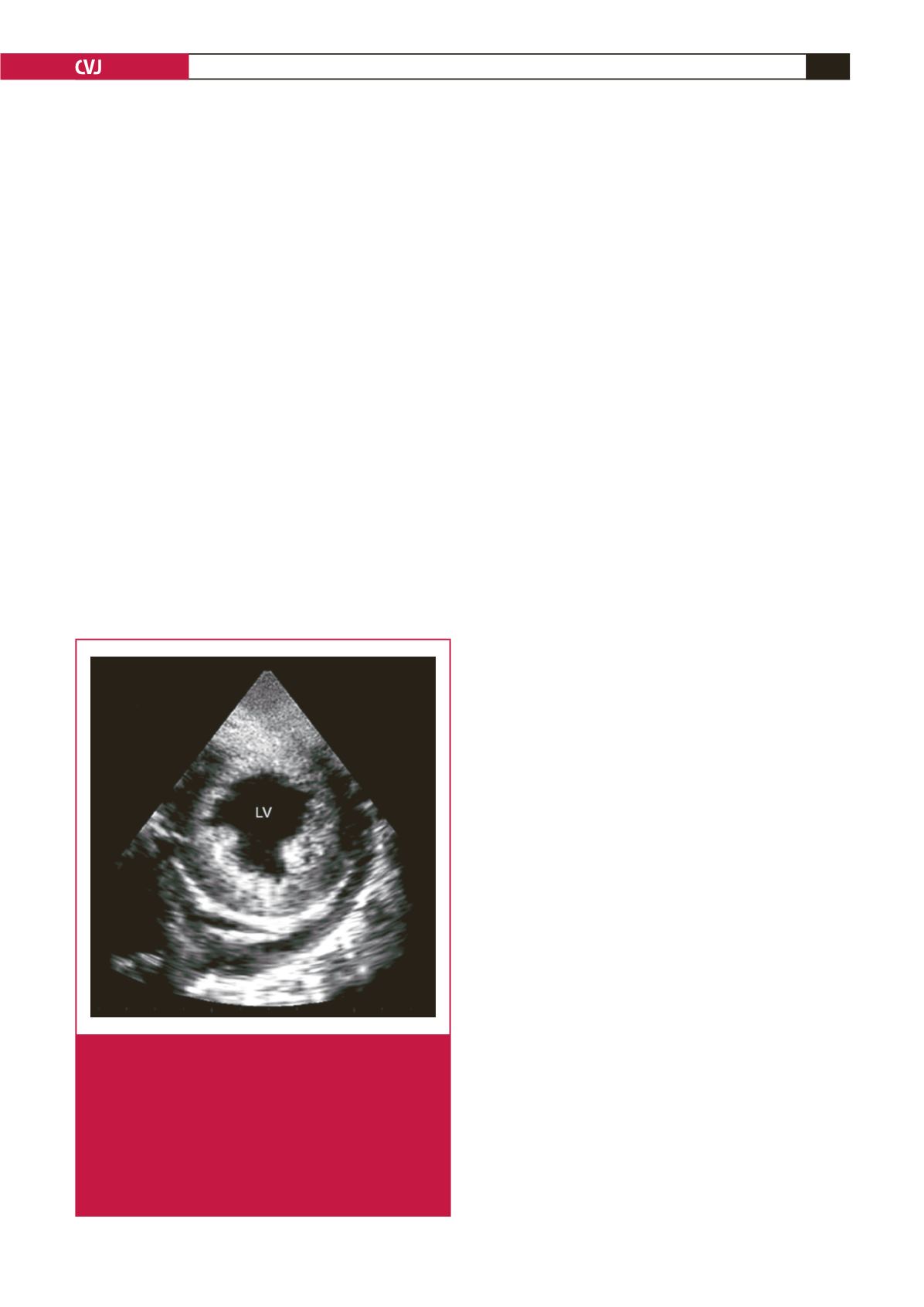
Fig. 6.
Endomyocardiopericardial fibrosis (EMPF). A short-
axis view from a patient with advanced EMF and
intractable heart failure. The endocardium looks dense
and bright with the myocardium clearly seen under-
neath it. The striking finding is the appearance of
densely fibrosed and calcified pericardium with effu-
sion forming an endomyocardiopericarial fibrosis.
Note the presence of pericardial effusion and the
posterior papillary muscle being engulfed by the thick-
ened endocardium.

















