
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 28, No 6, November/December 2017
364
AFRICA
aspirated. The subsequent pellet was washed twice with medium
buffer without substrate and then dissolved in 0.5 M NaOH; 50
μ
l of this solution was used for the determination of the protein
content by the method of Lowry
et al
.,
36
while the rest was
counted for radioactivity using a scintillation counter (Beckman).
The Western blot technique was performed as previously
reported, using the whole heart tissue
33
and isolated
cardiomyocytes.
34
Cell lysates were made after 30 minutes’
incubation with or without insulin or melatonin (before the
addition of 2DG). Thereafter the cells were put on ice, transferred
to Eppendorf tubes, quickly centrifuged and washed three times
with ice-cold medium buffer without substrate. The resultant cell
pellet was then lysed in 100
μ
l of lysis buffer.
34
At this point the
cells were sonicated on ice (three times, intervals of three-second
pulses with one-second break) and centrifuged for 20 minutes.
The subsequent pellet was discarded and the supernatant used
as cell lysate for Western blotting.
Total and phospho PKB/Akt (Ser-473) expressions were
evaluated in the cardiomyocytes after incubation with melatonin
with or without insulin, as previously described.
34
In addition,
GLUT4 expression was evaluated in whole heart lysates after
six weeks of melatonin treatment, as previously described.
33
All
antibodies were purchased from Cell Signaling (USA). Beta-
tubulin was used as a loading control. Protein activation is
expressed in arbitrary densitometry units as phospho/total ratios.
Statistical analysis
Data are expressed as mean
±
standard error of the mean (SEM).
When comparisons between two groups (treated and untreated)
were made, an unpaired Student’s
t
-test was performed. For
multiple comparisons, the ANOVA (two-way when appropriate),
followed by the Bonferroni correction was applied. Statistical
significance was considered for a
p
-value
<
0.05.
Results
Effect of melatonin treatment
in vitro
on glucose
uptake by cardiomyocytes
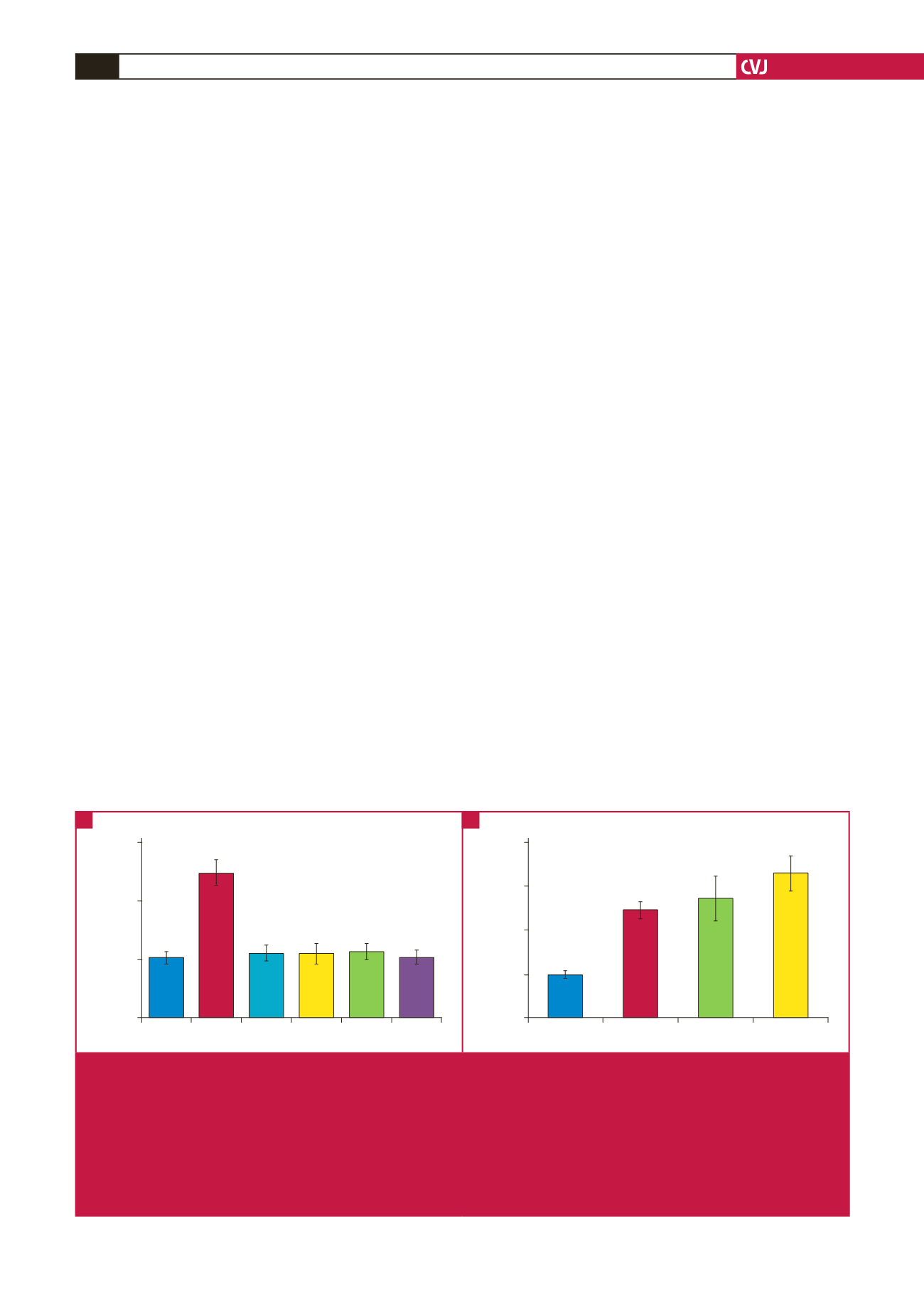
Compared to basal levels, melatonin treatment (10 and 100
nM, 10 and 50
μ
M) had no significant effect on glucose uptake
by the cardiomyocytes isolated from normal rats (Fig. 1A).
Insulin (1 nM) administration alone caused a 2.3-fold increase
in glucose uptake compared to basal levels (Fig. 1B). However,
when insulin was added to cells treated with melatonin (100 nM),
there was a further stimulation of glucose uptake (3.4
±
0.5- vs
2.5
±
0.2-fold increase,
p
<
0.05) (Fig. 1B). As melatonin at other
concentrations (10 nM) did not influence the levels of insulin-
stimulated glucose uptake (Fig. 1B) when compared to insulin
alone, only 100 nM was used in subsequent experiments.
Cardiomyocytes isolated from the control (C) and obese (D)
rats after 16 to 19 weeks of feeding, exhibited no significant
difference in basal as well as insulin-stimulated glucose uptake
between the two groups (Table 1, Fig. 2). As was observed in
cardiomyocytes isolated from normal rats (Fig. 1A), melatonin
administration (100 nM) also had no significant effect on basal
glucose uptake in group C and D rats fed for 16 to 19 weeks
(Table 1). However, it enhanced the insulin-stimulated glucose
uptake in group C compared to group D rats (C: 73.9
±
4.1 vs
D: 47.5
±
4.9 pmol/mg protein/30 min,
p
<
0.05) (Table 1, Fig. 2).
After 20 to 23 weeks of feeding, although the diet had
no significant effect on basal glucose uptake by isolated
cardiomyocytes (Table 2), insulin-stimulated glucose uptake was
significantly lower in group D rats compared with the control
group (C: 35.3
±
6.3 vs D: 25.9
±
1.6 pmol/mg protein/30 min,
p
<
0.05) (Fig. 3), while melatonin treatment had no effect on insulin-
stimulated glucose uptake in both group C and D rats (Fig. 3).
Basal
Ins Mel 1 Mel 2 Mel 3 Mel 4
60
40
20
0
2DG (pmol/mg protein/30 min)
**
Basal
Ins
Ins + Mel 1 Ins + Mel 2
4
3
2
1
0
2DG (fold stimulation)
**
*
Fig. 1.
Effect of
in vitro
melatonin treatment on basal and insulin-stimulated glucose uptake by cardiomyocytes from young control
rats (dose response). Cardiomyocytes were isolated and incubated with melatonin and/or insulin for a period of 30 minutes.
The accumulated radiolabelled 2 deoxyglucose (2DG) was measured using a scintillation counter and expressed as pmol/
mg protein/30 min. A: Effect on basal glucose uptake. Ins: insulin (1 nM), Mel: melatonin (Mel 1: 10 nM, Mel 2: 100 nM, Mel
3: 10
μ
M, Mel4: 50
μ
M), **
p
<
0.01 (vs basal or melatonin),
n
(individual preparations):
n
=
12 (basal), 11 (Ins), three (Mel 1),
eight (Mel 2), four (Mel 3), three (Mel 4); analysed in duplicate. B: Effect on insulin-stimulated glucose uptake (fold stimula-
tion). Ins: insulin (1 nM), Mel: melatonin (Mel 1: 10 nM, Mel2: 100 nM); *
p
<
0.05 (Ins vs Ins + Mel 2); **
p
<
0.05 (basal vs
Ins or Ins + Mel 1 or 2);
n
=
12 (basal), 11 (Ins), five (Ins + Mel 1), six (Ins + Mel 2) individual preparations/group; analysed
in duplicate.
A
B

















