
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 28, No 6, November/December 2017
AFRICA
367
Various physiological factors such as an effect on adiponectin
and leptin may have contributed to the overall effect of
in
vivo
melatonin on glucose uptake, as previously discussed.
10
In a preventative-treatment setting, 16 weeks of melatonin
consumption, starting before the establishment of obesity,
reduced hypertriglyceridaemia and increased high-density
lipoprotein cholesterol levels in rats fed the same high-calorie
diet.
32
However, the exact mechanism whereby
in vivo
melatonin
treatment affects glucose homeostasis and enhances insulin
responsiveness is complex and not fully elucidated.
Melatonin induced a significant reduction in body weight,
associated with a concomitant increase in basal glucose uptake
by isolated cardiomyocytes from the obese rats. This effect is
consistent with previous observations that chronic melatonin
treatment reduced body weight gain and insulin resistance in
mice
11
and rats
21
fed a high-fat diet, as well as in old obese
28
and
young Zucker diabetic fatty
13
rats. Therefore, melatonin action
may involve melatonin receptors and various indirect effects on
the liver, pancreas and other peripheral insulin-sensitive organs,
such as adipose tissue and skeletal muscle.
25
A recent report
shows that the removal of melatonin receptors (MT1 or MT2)
in mice abolished the daily rhythm in blood glucose levels,
44
confirming the role of melatonin signalling in the control of
glucose homeostasis.
Contrary to the
in vitro
situation, melatonin administered
in
vivo
increased basal glucose uptake by cardiomyocytes isolated
from obese rats. Mechanistically, this may involve glucose
transporter 1 (GLUT1), which is usually associated with basal
glucose uptake by cardiomyocytes, and its expression would give
more insight.
45
Therefore, it may be that there was an increase
in the expression or membrane translocation of GLUT1 in
these cardiomyocytes from obese rats treated with melatonin.
In addition, insulin was able to elicit a significant response in
untreated control animals, while this was not the case in the
obese animals after 20 to 23 weeks. This observation could
be explained by the insulin-resistant state of the cells from
the obese animals compared to their controls. Interestingly,
cardiomyocytes prepared from control as well as obese animals
treated with melatonin showed a significantly higher response to
insulin than the untreated counterparts (Fig. 4).
With regard to the effect of melatonin on glucose tolerance,
the present data show that obese rats developed glucose
intolerance, and melatonin had no effect on basal glucose levels
(10:00–12:00). While data on nocturnal glucose levels may be
different, six-week melatonin treatment also reduced systemic
insulin resistance in obese rats without affecting basal fasting
blood glucose levels.
33
These results are consistent with previous
findings:
46
between 15 and 25 minutes following glucose injection,
obese melatonin-treated rats had a significant decrease in blood
glucose levels compared to the untreated obese group, somehow
indicating their increased ability to absorb glucose.
The reduction in insulin resistance or improved glucose
uptake and utilisation may involve changes in the metabolic
profile, such as increasing adiponectin levels after long-
13,23
and short-term
33
melatonin administration. Melatonin-induced
beneficial changes in adipose tissue
41,47
may in turn additionally
contribute to improved whole-body insulin sensitivity. Moreover,
as indicated above, melatonin may improve glucose homeostasis
via its actions in the hypothalamus and liver.
48
Impairment of insulin-stimulatedglucose transport is considered
the most consistent change that develops early in the hearts of
animal models of insulin resistance.
26
Since GLUT4 is the most
prominent glucose transporter in differentiated cardiomyocytes,
49
our data underscore the importance of further investigation
analysing the expression of intermediates of insulin signal
transduction and the effects of melatonin treatment thereupon
in cardiomyocytes isolated from treated control and obese hearts.
The effect of six weeks of melatonin treatment on the basal
expression and activation of a number of intermediates in
myocardial tissue from control and obese rats has been studied
previously in our laboratory: baseline activation of PKB/
Akt, extracellular signal-regulated kinase (ERK) p42/p44 and
glycogen synthase kinase 3 beta (GSK3
β
) were found to be
significantly upregulated by melatonin treatment in both control
C
CM3
CM6
D
DM3
DM6
100000
80000
60000
40000
20000
0
100000
80000
60000
40000
20000
0
GLUT4 expression (arbitrary units)
GLUT4 expression (arbitrary units)
*
*
GLUT4
GLUT4
β
-tubulin
β
-tubulin
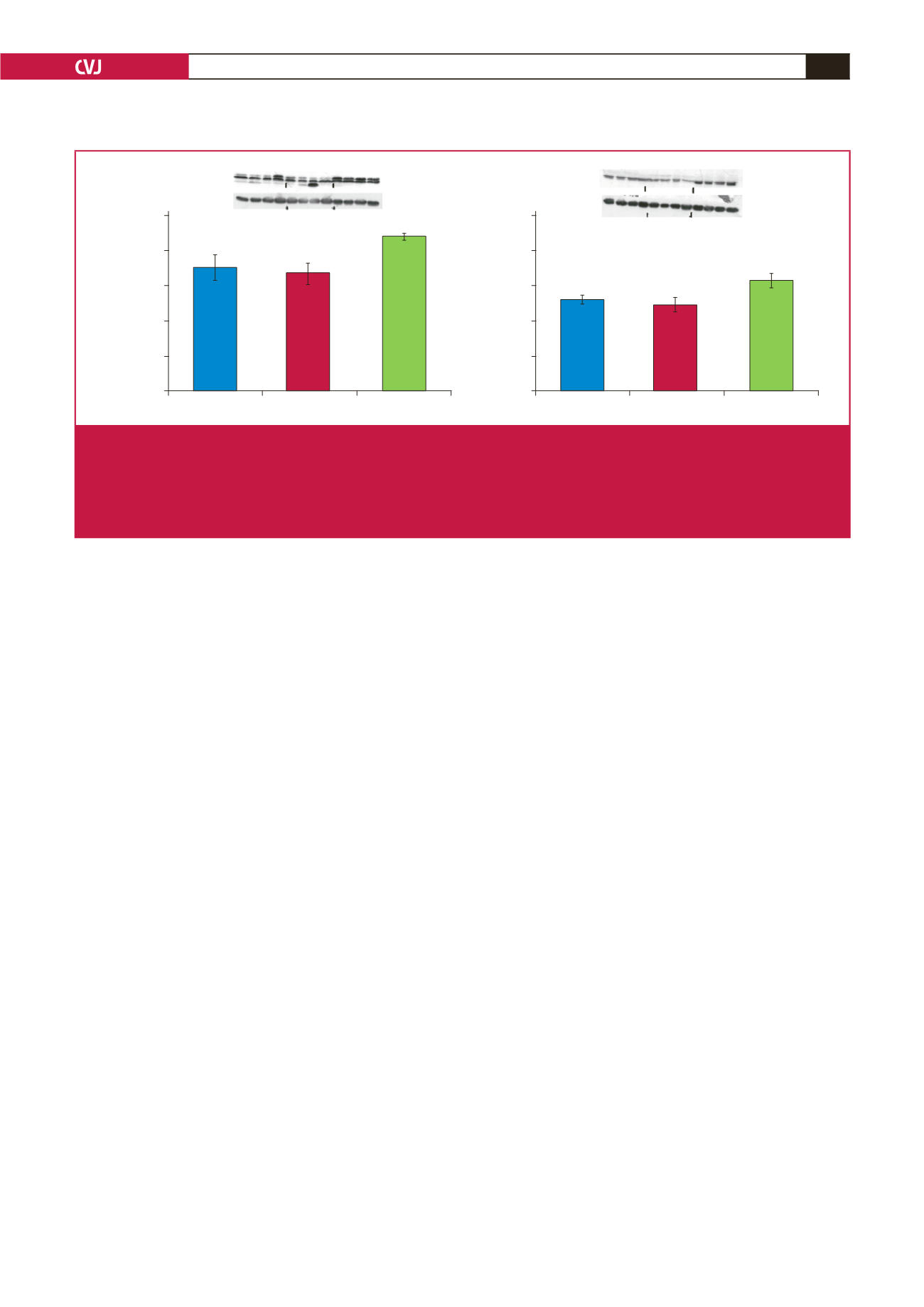
Fig. 6.
The effects of melatonin treatment on GLUT4 expression after three and six weeks of treatment. Hearts were isolated from
rats fed a high-calorie diet for 20 weeks and their age-matched controls. Both control and obese groups received drinking
water with/without melatonin (4 mg/kg/day) for three or six weeks starting after 14 weeks of feeding. C: control group, D: high-
calorie diet (obesity) group; CM3, DM3, CM6 and DM6: group C and D rats receiving melatonin treatment for three weeks
(M3) or six weeks (M6); beta-tubulin was used as a loading control. C and D performed on the different blot (
p
>
0.05 C vs
D), *
p
<
0.05 (CM6 vs C) or DM6 vs D,
n
=
four hearts/group.

















