
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 28, No 6, November/December 2017
366
AFRICA
(100 nM), compared to the untreated group (DM: 50.1
±
1.7 vs
D: 32.1
±
5.1 pmol/mg protein/30 min,
p
<
0.01) (Fig. 4).
Effect of melatonin treatment
in vivo
on IPGT test
in insulin-resistant rats
A high-calorie diet increased basal fasting blood glucose levels
compared to the control diet (5.2
±
0.28 vs 6.4
±
0.17 mM,
p
<
0.05). Similarly, at the end of the test, group D rats continued
to have elevated glucose levels (4.5
±
0.2 vs 5.2
±
0.1 mM,
p
<
0.05), compared to the control group (Fig. 5). The area under the
curve was also elevated in group D rats, compared to the controls
(870.7
±
25.6 vs 761.8
±
27.7,
p
<
0.05) (Table 3). However,
despite a significant decrease in blood glucose levels in the
melatonin-treated D rats observed between 15 and 25 minutes
of the test, we noted that melatonin treatment had no significant
effect on basal glucose levels and the overall area under curve in
both groups (Fig. 5).
Discussion
Our aim was to investigate the effect of melatonin treatment
on basal glucose uptake and insulin responsiveness as indicated
by glucose uptake, using cardiomyocytes isolated from young
control rats, age-matched controls and obese, insulin-resistant
rats. The results indicated that (1) melatonin treatment
in vitro
had no effect on glucose uptake but increased insulin-stimulated
glucose uptake by cardiomyocytes from only the young and
age-matched control rats (Fig. 1B, Table 1); (2) melatonin
treatment
in vivo
increased basal and insulin-stimulated glucose
uptake by cardiomyocytes isolated from the hearts of obese,
insulin-resistant rats.
During the basal state, glucose transport is commonly
considered the rate-limiting step for muscle glucose metabolism.
37
The involvement of melatonin in glucose uptake was supported
by the observation that pinealectomised animals develop insulin
resistance associated with a decrease in glucose uptake by
adipose tissue.
15,38
Accordingly, administration of melatonin
reversed pinealectomy-induced insulin resistance and improved
glucose uptake by isolated adipose tissue.
15,38
In contrast to this,
our data show that melatonin per se had no significant effect on
in vitro
glucose uptake by cardiomyocytes isolated from young
normal or obese rats and their age-matched controls (Fig. 1A,
Tables 1, 2). A similar observation was previously reported in
rat skeletal muscle cells
39
and chick brain,
40
as well as in adipose
tissue from a female fruit bat.
41
Of interest was our finding that acute melatonin
administration
in vitro
enhanced insulin-stimulated glucose
uptake by cardiomyocytes from normal young rats (Fig 1B)
as well as the control rats fed for 16 to 19 weeks (Fig. 2). The
enhanced insulin responsiveness of glucose uptake may be
related to a synergistic interaction between melatonin and insulin
action, supporting the insulin-sensitising effect by melatonin, as
previously demonstrated.
39,41,42
The
in vitro
melatonin-enhancing effect on insulin-stimulated
glucose uptake was not observed in cardiomyocytes isolated from
either the control or obese groups fed for more than 20 weeks
(Fig. 3), indicating a progressive loss of the synergistic interaction
between melatonin and insulin action. Although this is difficult
to explain, it may have resulted from ageing in the control group,
as previously demonstrated.
43
On the other hand, cardiomyocytes
from obese animals fed for 16 to 19 weeks were almost as insulin-
responsive as the control cardiac myocytes, but did not exhibit the
potentiating effect of melatonin compared to the control group.
Basal
Ins (1 nM)
Ins (10 nM) Ins (100 nM)
80
60
40
20
0
2DG (pmol/mg protein/30 mins)
*
**
#
##
##
##
##
##
###
**
*
&
###
###
#
++
++
C
D
CM
DM
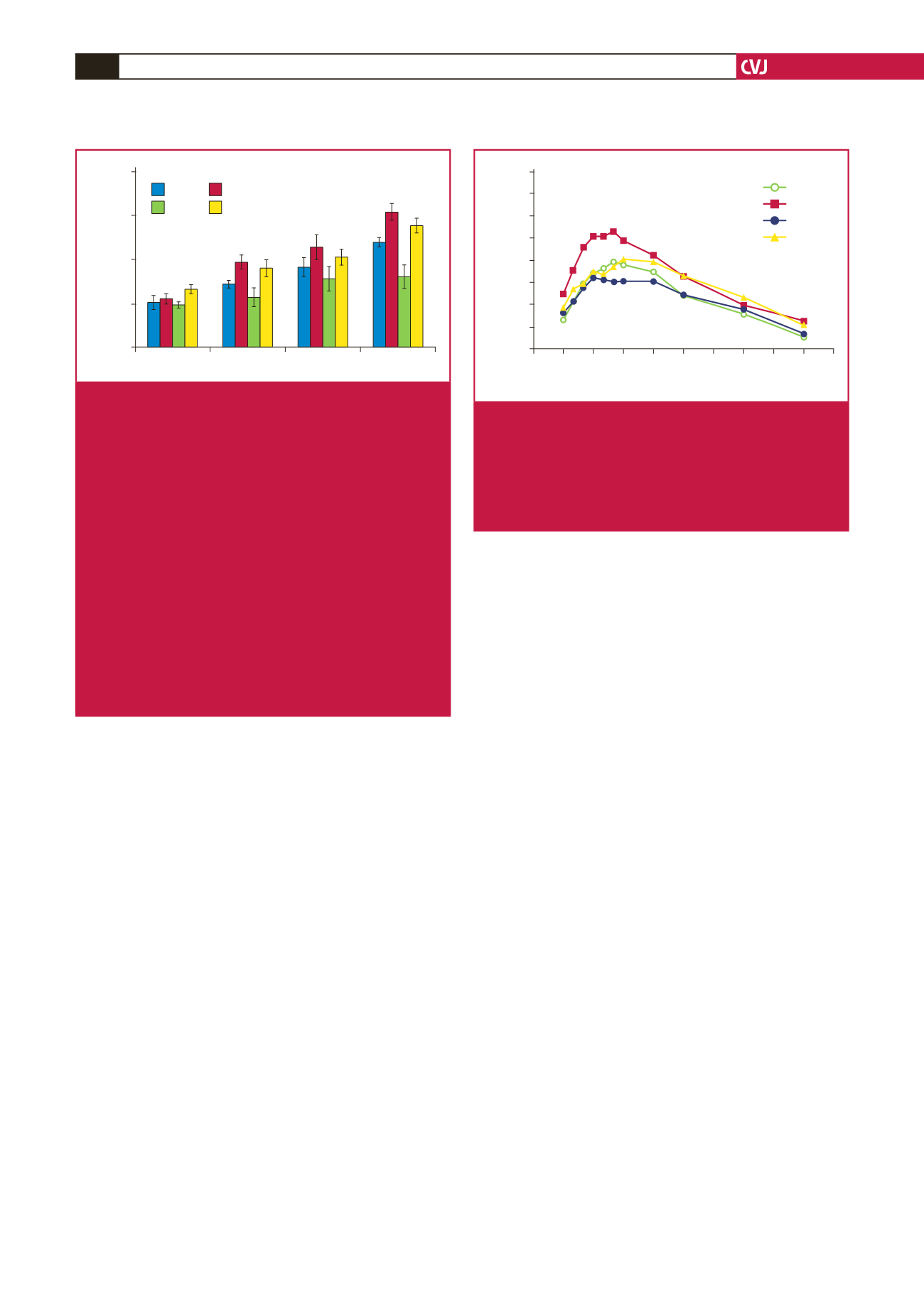
Fig. 4.
Effect of
in vivo
melatonin treatment (for the last
six weeks of feeding) on insulin-stimulated glucose
uptake by cardiomyocytes isolated from rats fed a
high-calorie diet (20 weeks). Cardiomyocytes were
isolated and stimulated with increasing concentrations
of insulin for a period of 30 minutes. The accumulated
radiolabelled 2DG was measured and expressed as
pmol/mg protein/ 30 min. Ins: insulin, C: control, CM:
control with melatonin, D: high-calorie diet (diet-
induced obesity), DM: diet with melatonin. Treated vs
untreated (same dose of insulin or basal): *
p
<
0.05
(DM vs D), **
p
<
0.01 (CM vs C). Different doses of
insulin vs basal (same group of treatment):
#
p
<
0.05
vs basal,
##
p
<
0.01 vs basal,
###
p
<
0.001 vs basal.
C vs D (same dose of insulin): and
p
<
0.05 (D vs
C). Comparison between different doses of insulin
(same group of treatment):
++
p
<
0.01 vs 1 nM Ins,
n
=
four to six individual preparations/group; analysed
in duplicate.
0
30
60
90
120
Time (min)
12
11
10
9
8
7
6
5
4
2DG (pmol/mg protein/30 mins)
C
D
CM6
DM6
*
*
*
#
Fig. 5.
Effect of
in vivo
melatonin treatment (for the last six
weeks of feeding) on intraperitoneal glucose toler-
ance. C: control, CM6: control with six weeks’ mela-
tonin treatment, D: high-calorie diet (diet-induced
obesity), DM6: high-calorie diet with six weeks’ mela-
tonin treatment, *
p
<
0.05 (D vs C),
#
p
<
0.05 (D vs
DM6),
n
=
six per group.

















