
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 28, No 6, November/December 2017
368
AFRICA
and obese rats.
33
However, it will be also important to determine
whether these observed beneficial changes were secondary to the
improved whole-body insulin sensitivity or whether there were
changes in cardiomyocyte protein expression and activation per
se elicited by melatonin treatment.
In this regard, a marginal increase in GLUT4 expression was
previously reported to be associated with an increase in glucose
uptake by melatonin-treated adipose tissue.
41
Our additional
observations showed significant increases in GLUT4 expression
in the whole heart tissue of obese rats after six weeks of
in
vivo
melatonin treatment (Fig. 6). Interestingly, as expected, the
significant lowering in glucose uptake by cardiomyocytes from
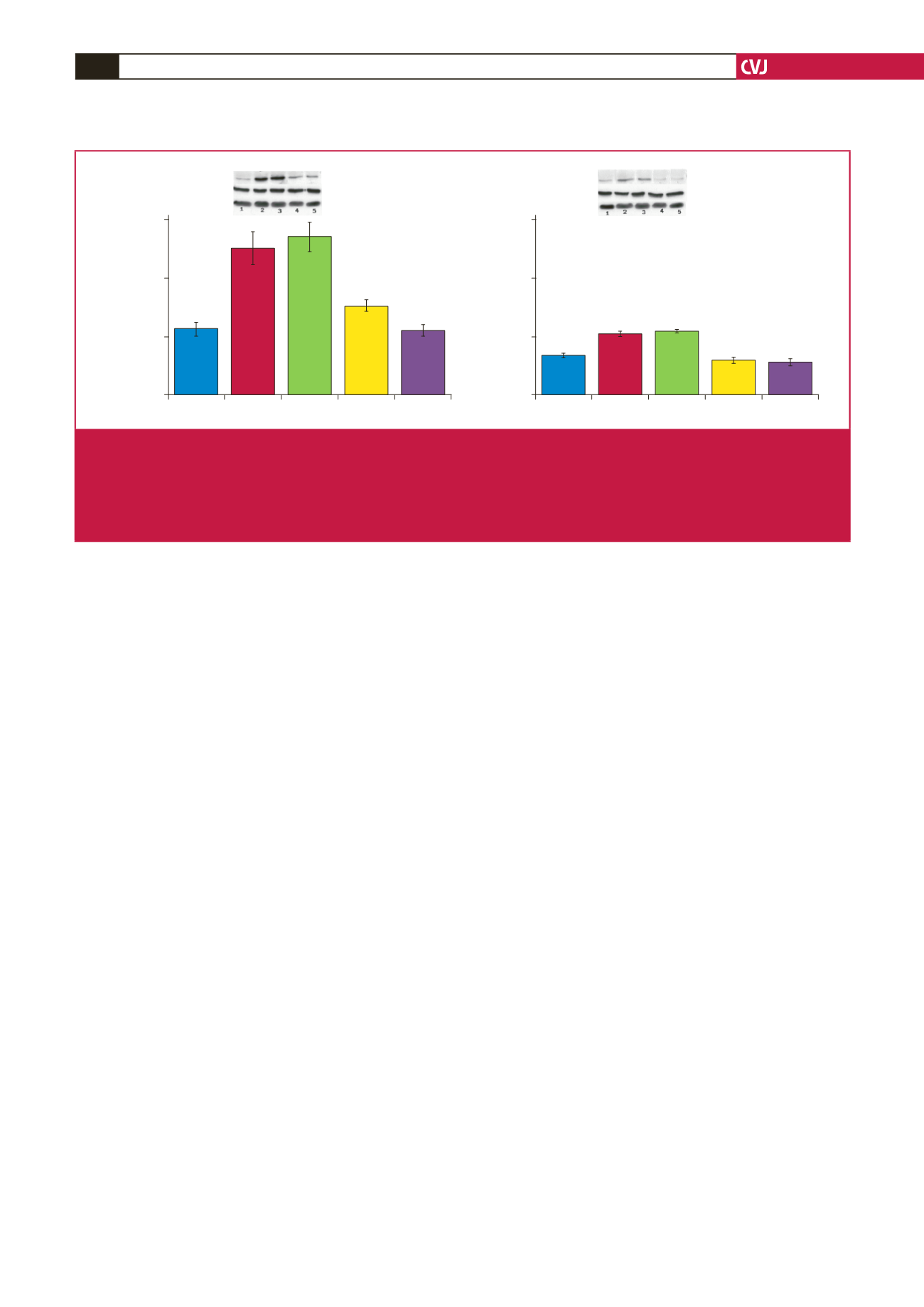
obese rats was also reflected in the reduction in PKB/Akt activation
when compared with their age-matched controls (Fig. 7).
Conclusion
To our knowledge, this is the first study on the role of
melatonin in cardiac glucose uptake in an insulin-resistant
state. The cardiovascular benefits of melatonin supplementation
are supported by the fact that circulating melatonin levels are
decreased in cardiovascular diseases.
50,51
Convincing evidence
exists for the benefits of increasing glucose uptake as an
important therapeutic goal in the management of left ventricular
systolic dysfunction.
52
Although its role in melatonin-induced
cardioprotection needs further investigation, present data
suggest that short-term melatonin treatment
in vivo
,
but not
in
vitro
, improved basal glucose uptake and insulin responsiveness
in insulin-resistant cardiomyocytes isolated from obese rats.
This study was supported by the SouthAfricanNational Research Foundation,
the Harry Crossley Foundation and Stellenbosch University.
References
1.
Muzigaba M, Puoane T, Sanders D. The paradox of undernutrition
and obesity in South africa: A contextual overview of food quality,
access and availability in the new democracy. In: Caraher M, Coveney
J, eds.
Food Poverty and Insecurity: International Food Inequalities.
UK:
Springer; 2016: 31–41.
2.
Finucane MM, Stevens GA, Cowan MJ,
et al.
National, regional,
and global trends in body-mass index since 1980: Systematic analysis
of health examination surveys and epidemiological studies with 960
country-years and 9·1 million participants.
Lancet
2011;
377
: 557–567.
3.
Rybnikova NA, Haim A, Portnov BA. Does artificial light-at-night
exposure contribute to the worldwide obesity pandemic?
Int J Obes
(Lond)
2016. [Epub ahead of a print].
4.
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH.
The incidence of co-morbidities related to obesity and overweight: A
systematic review and meta-analysis.
BMC Public Health
2009;
9
: 88.
5.
Scheen AJ, Van Gaal LF. Combating the dual burden: Therapeutic
targeting of common pathways in obesity and type 2 diabetes.
Lancet
Diabetes Endocrinol
2014;
2
: 911–922.
6.
Reaven GM. Insulin resistance: The link between obesity and cardiovas-
cular disease.
Med Clin North Am
2011; 95: 875–892.
7.
Benito M. Tissue specificity on insulin action and resistance: Past to
recent mechanisms.
Acta Physiol (Oxf)
2011; 201: 297–312.
8.
Riehle C, Abel ED. Insulin signaling and heart failure.
Circ Res
2016;
118
: 1151–1169.
9.
Hardy OT, Czech MP, Corvera S. What causes the insulin resistance
underlying obesity?
Curr Opin Endocrinol Diabetes Obes
2012; 19: 81–87.
10. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic
syndrome: A tool for effective therapy in obesity-associated abnormali-
ties?
Acta Physiol (Oxf)
2012; 205: 209–223.
11. Sartori C, Dessen P, Mathieu C,
et al
. Melatonin improves glucose
homeostasis and endothelial vascular function in high-fat diet-fed
insulin-resistant mice.
Endocrinology
2009; 150: 5311–5317.
12. Peschke E, Frese T, Chankiewitz E,
et al
. Diabetic goto kakizaki rats
as well as type 2 diabetic patients show a decreased diurnal serum
melatonin level and an increased pancreatic melatonin-receptor status.
J
Pineal Res
2006; 40: 135–143.
13. Agil A, Rosado I, Ruiz R, Figueroa A, Zen N, Fernandez-Vazquez G.
Melatonin improves glucose homeostasis in young zucker diabetic fatty
rats.
J Pineal Res
2012;52: 203–210.
Basal
Ins Ins+Mel
Mel
Mel+Luz
Basal
Ins Ins+Mel
Mel
Mel+Luz
1.5
1.0
0.5
0.0
1.5
1.0
0.5
0.0
p-/total PKB/Akt (ser-473) ratio
(arbitrary units)
p-/total PKB/Akt (ser-473) ratio
(arbitrary units)
*
*
&
#
Total PKB/Akt
Total PKB/Akt
p-PKB/Akt (Ser-473)
p-PKB/Akt (Ser-473)
β
-tubulin
β
-tubulin
Fig. 7.
Effects of
in vitro
melatonin administration to isolated cardiomyocytes on PKB/Akt expression and phosphorylation (rats fed
for 20 weeks). Cardiomyocytes were isolated and incubated with melatonin with or without insulin stimulation. C: control, D:
high-calorie diet. 1: basal, 2: Ins (insulin), 3: Insulin + melatonin, 4: Mel (melatonin), 5: luzindole + melatonin, Luz (luzindole),
C: *
p
<
0.05 (Ins or Ins + Mel vs basal), and
p
<
0.05 (Mel vs basal or Mel + Luz), D: *
p
<
0.05 (Ins or Ins + Mel vs basal),
#
p
<
0.05 (D vs C),
n
=
three individual preparations/group. Blots are representative. Beta-tubulin was used as a loading control.
C and D performed on the same blot.

















