
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 32, No 2, March/April 2021
100
AFRICA
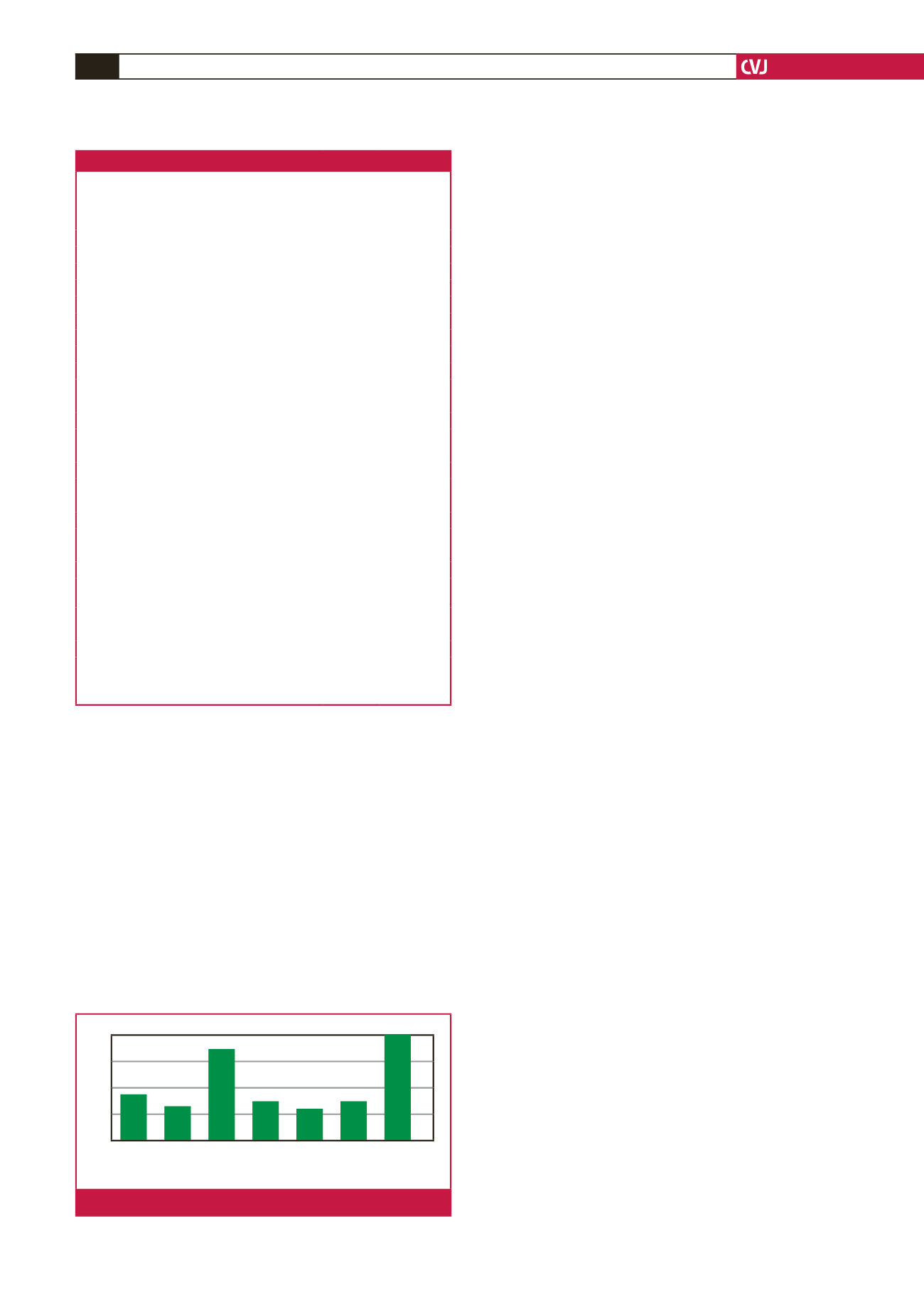
While late presentation with advanced kidney disease is a
common occurrence in our unit, necessitating the use of CVCs,
long delays to creation of permanent access after starting dialysis
prolongs exposure to the harmful effects of CVCs.
11,20
Almost a
third of patients in this study waited more than 12 months prior
to the first AVF attempt. This most likely contributed to the high
failure rate when an AVF was eventually created. Pre-emptive
fistulae should ideally be fashioned three to six months before
the first haemodialysis session to allow for maturation and
re-intervention if necessary.
5-7
These findings are similar to reports from sub-Saharan Africa
as well as other low- and middle-income countries, where most
patients will start dialysis on an emergency basis and cannot wait
for a fistula to mature.
16,21
This perpetuates the cycle, as higher
rates of CVC use lead to poorer outcomes with AVFs, which lead
to more CVC use. Except for the high primary failure rate, a lack
of secondary intervention also decreases the long-term patency
rates of the AVFs. Whenever failing fistulae are identified,
rapid referral for intervention prior to a complete occlusion is
required. Interventions done to maintain the fistula prior to
complete occlusion are more likely to be successful.
22
The access
surgeon also requires available theatre time to be able to attempt
salvage. When urgent secondary interventions are not available,
the fistulae will simply be abandoned when they occlude.
16
The documented complication rates may have been
underestimated in this retrospective study as it relied on the
adequacy of the patient records. Central venous stenosis or
occlusion was recorded in a quarter of the patients. This may
even be an underestimation, since patients are not routinely
screened for evidence of central venous obstruction and only
clinically apparent central venous obstruction was recorded. The
damage caused by long-term CVC use leads to central venous
stenosis and can compromise future access options.
23
Recommendations for improving current practice
Early detection of CKD and timely referral: many patients
present late with end-stage kidney disease. Ongoing education of
healthcare providers is needed to promote early referral. Early
detection of CKD may avoid the need for urgent dialysis and
therefore CVC use, allowing time for pre-emptive access creation.
24
Dedicated vascular access clinic: a specialisedmultidisciplinary
clinic should be formed that deals primarily with new and
problematic vascular access cases.
18
This multidisciplinary team
should include a vascular access surgeon, a nephrologist, dialysis
nursing staff and supporting staff. All new referrals can be seen
and access planning started prior to the first dialysis session.
Ultrasound evaluation can be done at the initial visit to map
out potential access sites and look for problematic areas such
as prior vein injury by cannulation. When there are concerns
regarding early AVF failure, intervention can then be planned
and the patient prioritised for surgical revision from this clinic.
In this format there will be open communication between the
different members of the haemodialysis team. It will also allow
time for patient education in a neutral environment with all the
different team members available.
Availability of a dedicated vascular access theatre list: without
access to theatre it would not be possible to run an effective
vascular access service. The best way to optimise the timing to
AVF creation and deal with failing fistulae or complications
would be to allocate a dedicated vascular access theatre list.
This list should ideally be in a hybrid theatre or a theatre
with fluoroscopy available so that both open surgical and
endovascular interventions can be performed as needed. The
haemodialysis patients can then be prioritised and would not
need to compete for theatre time with all the other emergency
and elective surgical patients.
A dedicated access co-ordinator: it would be valuable to
appoint a dedicated vascular access co-ordinator. This should
be a trained nurse experienced in haemodialysis and vascular
surgery. Ideally one of the experienced nurses currently in the
unit could fulfil this role. The co-ordinator will be the link
between the patient, dialysis staff, nephrologist and access
surgeon. This strategy has been shown to be very effective in
improving haemodialysis outcomes.
18
Table 2. History of vascular access creation and complications
History
Number
Percentage of
study
populations
Current access
Tunnelled CVC
37
56
AVF
25
38
AVG
3
5
Temporary CVC
1
2
CVC group sub-analysis
Patients using a CVC at present
37
56
With no previous AVG or AVF
8
12
With 1 previous AVG or AVF
14
21
With > 1 previous AVG or AVF
15
23
Initial vascular access
CVC
63
95
Non-tunnelled CVC
38
58
Tunnelled CVC
25
38
Pre-emptive AVF
3
5
Number of AVF or AVG attempts
No previous AVF or AVG
8
12
1 AVF or AVG
29
44
2 AVF or AVG
15
23
3 AVF or AVG
13
20
4 AVF or AVG
1
2
Complications
Central venous stenosis or occlusion (of 66
patients)
17
26
Aneurysmal dilatation (of 101 AVFs)
15
15
Aneurysmal and still in use (of 15)
9
60
Aneurysmal and abandoned (of 15)
6
40
Dialysis access-associated steal syndrome
0
0
CVC, central venous catheter; AVF, arteriovenous fistula; AVG, arteriovenous
graft.
18
14
9
5
0
Time to first AVF attempt in relation to starting haemodialysis (months)
No attempt Pre emptive 0–3 3–6 6–9 9–12
>
12
Fig. 1.
Time to first fistula attempt (months).



















