CARDIOVASCULAR JOURNAL OF AFRICA • Vol 23, No 10, November 2012
AFRICA
559
programmes/induces them to attack the patient’s tissues and
organs and cause the fibrotic reactions seen in the hyper-
eosinophilia of Löffler’s disease?
2.
Can filaria worms induce eosinophils to proliferate on a
massive scale (i.e. hypereosinophilia), infiltrate major organs
of the body, degranulate and attack patients’ tissues and
organs during the process of eliminating the invading organ-
isms?
3.
Are there other agents – toxins, infections such as
Toxoplasma
gondii
and
Schistosoma mansoni –
that are capable of damag-
ing the endomyocardium like hypereosinophilia?
4.
Can parasites such as filaria worms, ova of common parasites
such as
Schistosoma,
or other organisms such as
Toxoplasma
gondii
get caught within the endomyocardium, cause chronic
inflammation and subsequently fibrotic reactions? These
parasites are known to lodge in various organs of the body
such as the liver and lungs, where they induce fibrotic reac-
tions, and it is often forgotten that they can also lodge within
the myocardium of the heart. There may be an eosinophilic
reaction to the parasite in such a situation but this will be
mild and transient, and not on the same scale as Löffler’s
endomyocardial disease.
5.
Is the peculiar location of the lesions in EMF and EED due to
the mode of blood flow through the ventricles? Studies have
shown that there is relative stasis of blood flow within the
apices of the ventricles and for this reason most clots congre-
gate at the apices of the ventricles.
6.
Is there a consensus with the pathogenesis proposed by
Olsen,
81
of fibrotic lesions within the endomyocardium of
patients with EMF/EED? His studies showed that the process
of development of EMF/EED goes through the following
phases:
––
necrotic phase with active myocarditis, inflammatory infil-
trates and eosinophils
––
thrombotic phase with endocardial thickening, thrombosis
and decrease in the number of inflammatory cells
––
fibrotic phase involving the endocardium, replacement of
tissues by collagen and superficial thrombosis.
7.
What is the implication of the recent observations in Uganda
by Freers
et al
.,
82-84
which showed that fibrosis in patients
with EMF is not confined to the endomyocardium but also
occurs in other tissues such as the peritoneum, pleura, liver
and pericardium. Does it conform to the parasitic theory put
forward in (4) above?
8.
Are there others markers specific for Löffler’s endomyo-
cardial disease that could make the diagnosis of the disease
easier in Africa?
Answers to these questions will help researchers in Africa
make significant progress in finding the cause(s) of EMF. The
greatest problem we have with the disease at present is that we
do not know how the illness begins. Several investigators have
described what they believe are the early illnesses of the disease
but these are not convincing. Yet, it is during the early stages of
the disease that we can find its cause and define its pathogenesis.
The EMF cases seen in our wards in Africa are already in
the chronic stages of the disease, when the cause is difficult
or impossible to find. By going to the villages where there
is poverty and a high incidence of EMF, and by using an
echocardiogram to aid their study, the research team presently
working in Mozambique
85
has a unique opportunity to help us
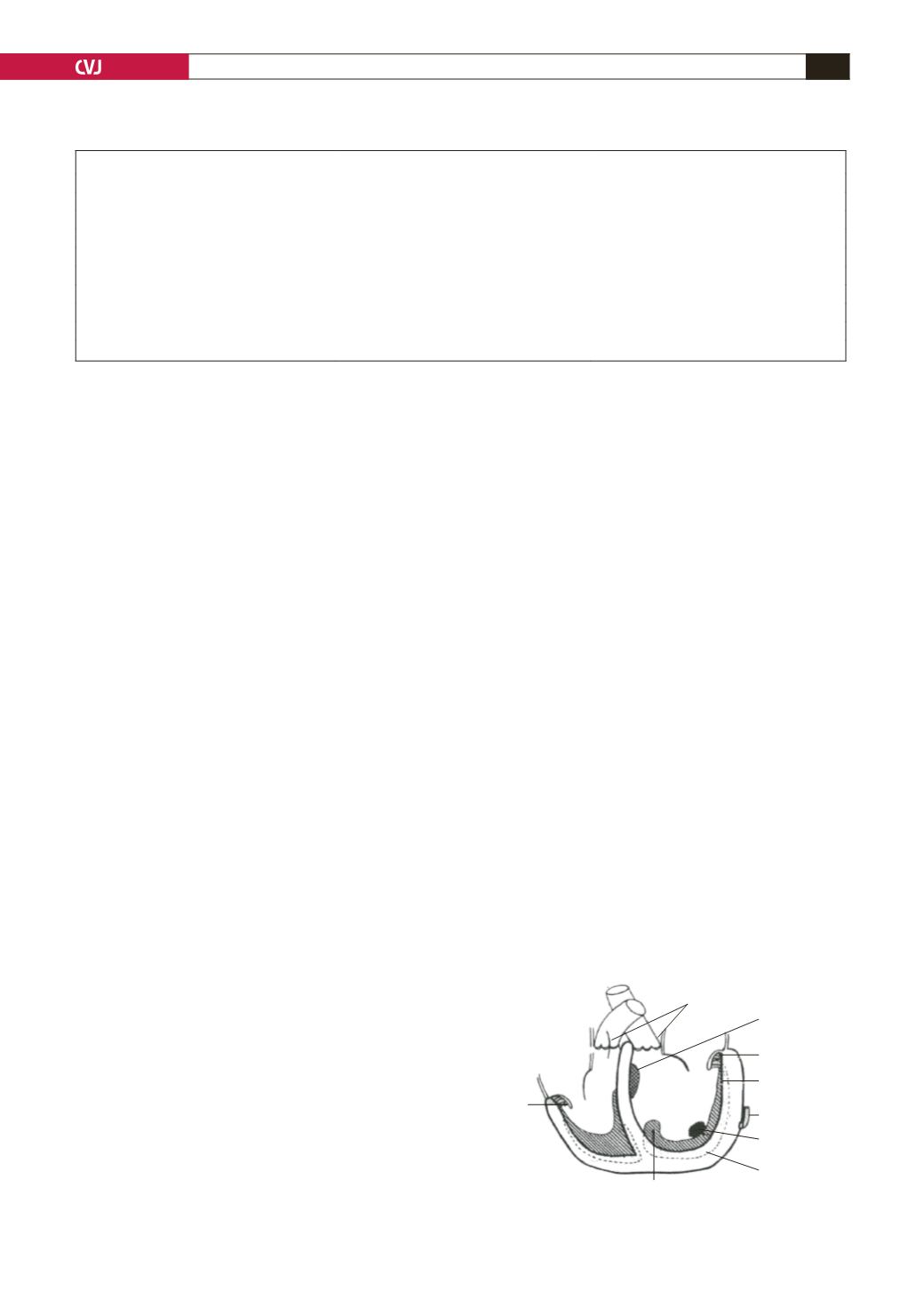
find the cause of the disease (Fig. 5).
Left ventricular non-ischaemic ventricular aneurysms
These are ventricular aneurysms that occur in black people living in
the tropics, particularly those who live in the equatorial rainforest
belt of Africa, and are not due to or associated with coronary
artery occlusion.
86-88
They belong to the group of forgotten tropical
cardiomyopathies. Although they are now rare, they still occur.
Sporadic cases of such aneurysms have been described in the past
from the United States of America, France, Sweden, South Africa
and the West Indies, mainly among black people living in those
countries. It was first reported in 1813 by Corvisart, in a black man
who died of the disease in France in 1796.
The aneurysms are often sub-valvular in location, more
commonly affecting the mitral and aortic valve rings. Sub-mitral
aneurysms tend to be very large, often creating mitral
regurgitation; sub-aortic aneurysms on the other hand tend to
be smaller and may cause left and right ventricular outflow
TABLE 1 . DIFFERENCES BETWEEN TROPICAL EMFAND EED
Parameter
Tropical EMF
EED (Loeffler’s)
Constitutional symptoms
Absent
Present
Hypereosinophilic syndrome
Absent
Present
Degranulated and vacuolated eosinophils
Few cases
Invariably present
Cationic proteins
Not elevated
Elevated
Location of lesions
RV, LV or BV
Invariably BV
Geographical distribution
Mainly rainforest regions
Worldwide
Age group
Usually children
No specific age group
Eosinophilic infiltration of other organs
Absent
Present
EMF = endomyocardial fibrosis; EED = eosinophilic endomyocardial disease; RV = right ventricular; LV = left ventricular; BV = biventricular.
Fig. 5. Davies’ representation of endomyocardial fibrosis.
Tricuspid
posterior
cusp
Semilunar valves
uninvolved
Secondary
elastomyofibrosis
Mitral
posterior cusp
Fibrosed
endocardium
Normal
coronary artery
Mural thrombus
(41%)
Fibrosed
myocardium
Thick mass junction of
inflow–outflow tract
L.V.
R.V.
[
no infarctions, no arteritic lesions]


