
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 26, No 2, March/April 2015
AFRICA
73
aneurysms may be associated with venous thrombosis as a result
of venous compression by the aneurysmal mass.
Imaging studies
Studies have shown aneurysmal transformation of the carotid,
aortic, femoral and popliteal vessels.
3,4
These aneurysms are
multiple (usually more than three) (Fig. 2C) with a greater
frequency in the carotid and femoral vessels. Doppler studies
demonstrating the imaging features in HIV-associated
vasculopathy are documented uncommonly. Woolgar
et al
.
32
described the imaging characteristics with the aid of duplex
ultrasound in HIV-related aneurysms in 12 patients. This
modality has proven to be a valuable non-invasive screening tool.
As a general principle, aneurysms found clinically at one
location guided the screening for aneurysms at other locations.
Duplex ultrasound features characterised by forward and
backward flow within a pseudo-aneurysm are reflected as
a ‘yin-yang’ sign
32
(Fig. 2D), cavitational echogenicity and
turbulent flow with a vessel wall defect (Fig. 2E).
32
This
pathological process is eclipsed by adjacent hypo-echoic spotting
and vessel wall thickening with normal proximal and distal
vasculature. Patients deemed suitable for surgery are subjected to
definitive imaging in the form of angiography or computerised
tomographic angiography. Angiographically, the aneurysms are
usually multiple, saccular or pseudo-aneurysmal with a variable
location. The uninvolved arterial segments are pristine with a
smooth vessel contour (Fig. 2C).
Pathology
HIV-associated vasculopathy has a predilection for medium
and large vessels. Its pathological profile has been compared
to Takayasu’s disease because it affects young individuals
and shares similar disease distribution and transmural vessel
involvement. Histopathological vascular changes in AIDS were
initially described by Joshi
et al
.
5
in autopsies of children. Small
and medium-sized vessels in six children demonstrated intimal
fibrosis, elastic fragmentation, medial calcification, luminal
narrowing and perivasculitis.
5,33
By contrast, Calabrese
et al.
34
documented a systemic
necrotising vasculitis following HIV infection in 11/14 patients
with small-vessel involvement and lymphocytic infiltration.
These features overlapped with polyarteritis nodosa, angiocentric
lymphoproliferative disorder and primary angiitis of the nervous
system.
34
Marks and Kuskov
28
demonstrated peri-arteritic
fibroproliferative granulomatous inflammation of the aortic
and iliac vessels in 5/12 patients with HIV-associated aneurysms,
and hypothesised that most HIV patients develop a necrotising
vasculitis of the vessel wall followed by the development of false
aneurysms.
Some autopsy case studies and series of HIV-associated
intracranial aneurysms,
35-45
documented mainly in children, report
similar microscopic features to those in extracranial vessels.
While this observation suggests that intracranial aneurysmal
pathology is a continuum of the same disease process, some
workers, however, have noted differences as follows:
•
variable absence of internal elastic lamina fragmentation
•
medial thickening with sub-intimal SMC deposition
•
controversial identification of viral protein, specifically gp 41,
in the macrophages of the arterial wall, with inflammatory
sparing of smaller leptomeningeal and parenchymal vessels
•
absence of vasa vasora in the intracranial vessels, suggestive
of a mechanism other than a leucocytoclastic vasculitis
•
identification of specific vasculitic agents, such as varicella
zoster virus, in lesional tissue.
In our unit Nair
et al
.
29
studied the histopathological features of
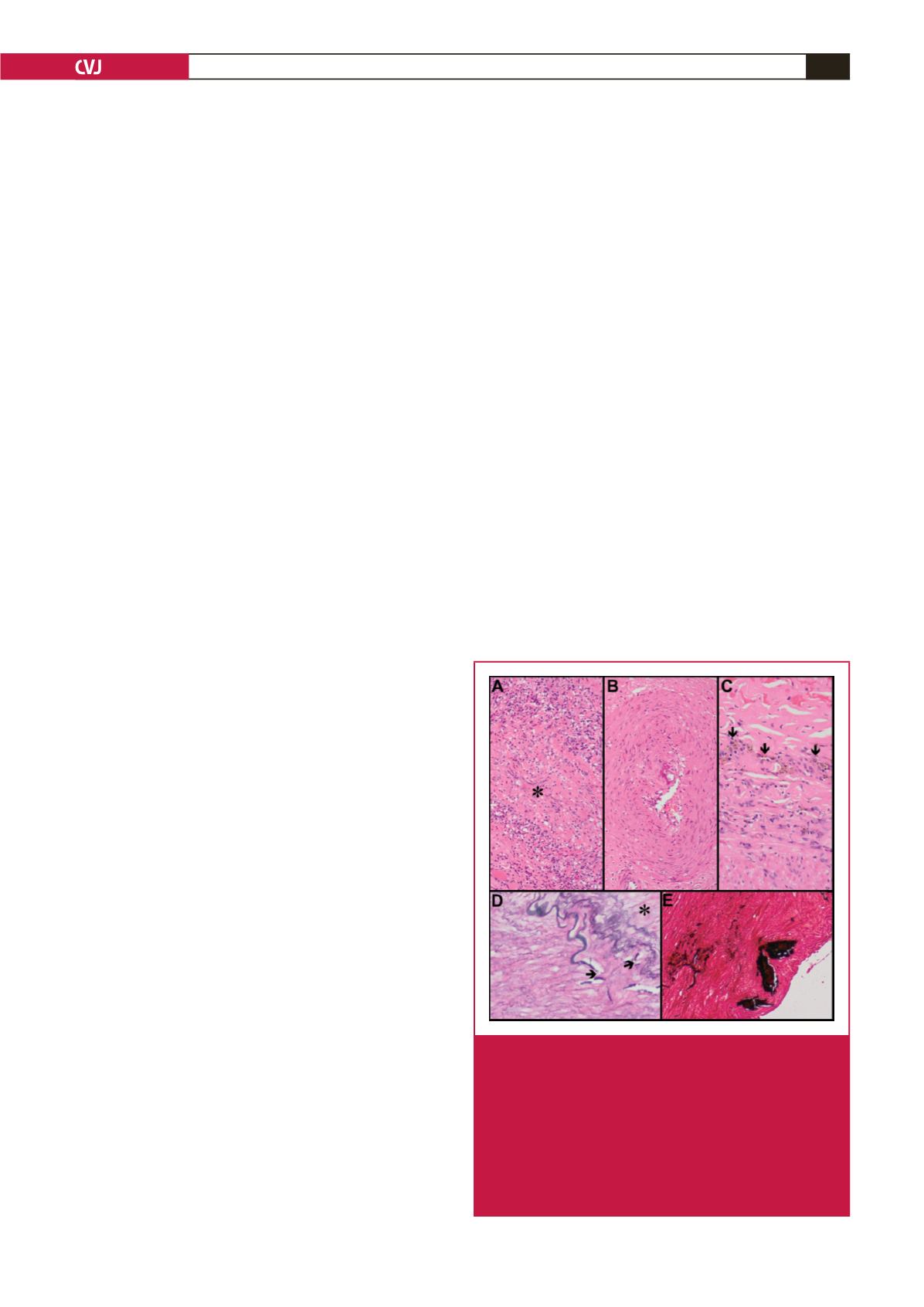
the aneurysm wall in detail in 10 patients. The common theme
that was documented was inflammation of the vessel walls
with the vasa vasora being the epicentre of this inflammatory
process. Active lesions demonstrate inflammatory changes,
necrosis and luminal narrowing (Fig. 3A), while inactive lesions
are characterised by chronic features, including fibrosis and
haemosiderin deposition. The media displays fragmented
elastic fibres, variable loss of smooth muscle and fibromuscular
hyperplasia (Fig. 3B). Intense involvement of the vasa vasora
is hypothesised to cause transmural ischaemic necrosis. The
adventitia demonstrates evolving inflammatory changes,
characterised by macrophage infiltration with haemosiderin
deposits.
Similarly, large-vessel vasculopathy has also been
characterised by adventitial, medial and intimal alterations.
6,46
Leucocytoclastic vasculitis of the vasa vasora and peri-
adventitial vessels, proliferation of slit-like vascular channels,
chronic inflammation and fibrosis are seen in the adventitia (Fig.
3C). While medial fibrosis, muscle damage, elastic fragmentation
and intimal duplication are also present, the intima demonstrates
fragmentation of the internal elastic lamina (Fig. 3D) and
calcification (Fig. 3E).
Fig. 3.
Histopathology of HIV aneurysmal disease: active
vasa vasorum inflammation (A), and luminal narrow-
ing (*) (haematoxylin and eosin, 240
×
); vasa vaso-
rum fibromuscular hyperplasia (B) (haematoxylin and
eosin, 240
×
); peri-adventitial slit-like vascular channels
with inflammatory cells (C); and haemosiderin pigment
(arrows) (haematoxylin and eosin, 240
×
). Vessel wall
(D) with fragmentation of the internal elastic lamina
(arrows) (*
=
intima) (elastic van Gieson, 240
×
) and
medial calcification (E) (von Kossa, 240
×
).

















