
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 26, No 2, March/April 2015
74
AFRICA
Bacterial infection is also hypothesised to play a minor
pathogenetic role. Although secondary infection caused by
bacteria, viruses and fungi have been implicated, this has not been
conclusively demonstrated. In the series by Nair
et al
.,
29
micro-
organisms were not identified, while in that of Marks and Kuskov,
28
Staphylococcus aureus
was isolated from peri-aneurysmal exudate.
It is debatable whether the latter was a surface contaminant.
Management
In the current era, HIV-infected patients presenting with vascular
pathology are managed by the standard guidelines of HIV-naïve
patients, with conservative management being reserved for
patients with full-blown AIDS.
3,4,26,47-49
The overall management
of these patients poses a moral and ethical dilemma with regard
to the appropriateness and timing of surgical intervention.
At present there are no universal guidelines. Emergencies are
prioritised irrespective of immune status. The majority of
patients are young, fit and able to tolerate major surgery.
Treatment should be individualised and priority given to
patients with symptomatic aneurysms. Intra-operatively, false
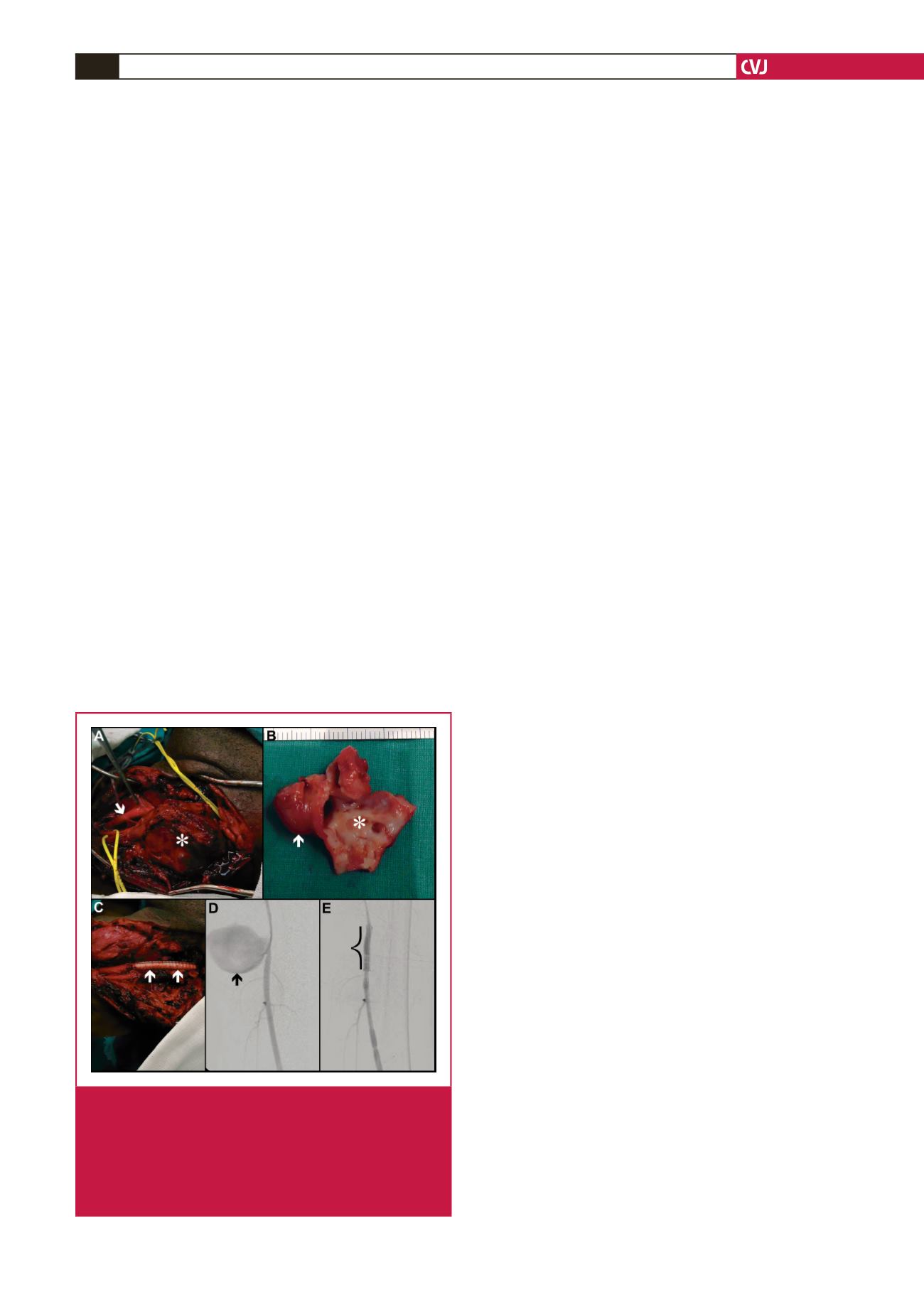
or true aneurysms are identified (Fig. 4A).
3,4,26
Intervention is
offered for symptomatic aneurysmal lesions, and involves either
ligation of vessels in septic lesions and occluded distal vessels, or
resection (Figs 4B) and restoration of arterial continuity (Fig.
4C) following aneurysmal excision.
3,4
The ligation of carotid
lesions seems to be well tolerated, as evidenced by Nair
et al
.,
29
with little or no neurological sequelae peri-operatively. The
choice of conduit, namely prosthetic or autogenous grafts, for
surgical bypass remains controversial.
29
The latter is plagued by
the associated risk of deep-vein thrombosis in these patients.
In selected patients with appropriate technical imaging criteria
and poor physiological reserves, endovascular management with
a stent-graft (Fig. 4D, E) constitutes a suitable alternative.
Anecdotal reports with small patient numbers have documented
its selected use and immediate success.
26,50,51
Complications of
this modality include stent-graft sepsis, occlusion, endoleaks and
missed opportunistic infections.
4,52
Scholtz
53
has raised concerns
about this modality, from a radiological perspective, with
regard to angiographic access, small-calibre vessels and contrast
pooling in the multiple aneurysms. Despite these reservations, it
remains an attractive alternative because it promotes flexibility
of treatment options. Endovascular intervention represents
technology in evolution, with unknown long-term results. Patient
selection is therefore crucial; the procedure should be reserved
for patients with poor physiological reserves.
There have been no comparative studies, to date, on
surgery versus endovascular intervention in patients with HIV
vasculopathy. Expertise in this sphere is at present anecdotal
and has been confined to isolated case reports.
54,55
From a
technical perspective, these aneurysms may present a challenge
in relation to their large size, vessel tortuosity, flow dynamics
and specific anatomical location, especially in relation to outflow
tracts that may originate in close proximity to, or from the
aneurysm sac itself. The branch vessels of interest in this instance
would include the vertebral, internal iliac and visceral arteries.
The rationale for endovascular intervention entails aneurysmal
exclusion of the target vessel and preservation of laminar flow
in the outflow tract. Endovascular devices to exclude these
aneurysms include the use of covered or uncovered stents in the
form of multi-layered compact cobalt,
55
or open-cell nitinol mesh
design. More recently, a novel idea of aneurysm exclusion using
multi-layered stent technology without compromising branch
vessel patency has been reported in HIV-infected patients.
The basis for this technique has been extrapolated from the
pipeline embolisation device,
54
which is used to treat intracranial
aneurysms. Its successful usage has been described by Euringer
et al.
55
in a patient with multiple HIV-related aneurysms. The
deployment of this type of stent achieves aneurysm exclusion
and restoration of laminar flow with aneurysm autothrombosis
as a result of strangulated flow.
This technique can, in theory, also be augmented with
coiling to ensure complete thrombosis within the aneurysm
sac. Success when utilising the multi-layered stent in this setting
requires definition because the aneurysmal sac can be completely
excluded with total sac content thrombosis or sac shrinkage
with sluggish flow. According to the authors,
55,56
the merit in this
technique facilitates continuous branch vessel patency, especially
the patency of visceral branches of the aorta. Sustained patency
may require the use of long-term antiplatelet therapeutic agents
such as clopidogrel. The effect of this type of anti-platelet
therapy may be negated by some anti-retroviral agents,
54
but it
is possible to identify patients with this form of resistance prior
to intervention.
The multi-layered stent has the technical limitations of a
compact strut design, which precludes additive coiling and
potential excessive foreshortening during deployment. This
may result in a geographical miss. Although the endovascular
approach is cushioned and not plagued by the physiological
challenges of robust anaesthesia, blood loss and the peri-operative
complications of contamination, wound sepsis and transfusion
Fig. 4.
HIV aneurysm management: intra-operative left
common carotid artery exposure (A, arrow) with
aneurysm (*). Resected specimen (B) with aneurysm
(arrow) and pseudo-aneurysm (*). Prosthetic inter-
positional graft (C, arrows). Endovascular manage-
ment of a left superficial femoral artery pseudo-aneu-
rysm (D, arrow) with a stent graft (E, bracket).

















