CARDIOVASCULAR JOURNAL OF AFRICA • Vol 21, No 1, January/February 2010
38
AFRICA
(PBS), cut into small pieces of about 1 mm
3
and digested with
0.1% trypsin (Sigma). Cells were harvested after digestion and
resuspended in Dulbecco’s modified Eagle medium (DMEM)/
F12 (1:1) (GIBCO) supplemented with 10% (v/v) foetal bovine
serum (FBS, Hyclone), 100 U/ml penicillin and 100
µ
g/ml
streptomycin. The fibroblast content in the cell suspension was
reduced using a differential attachment method.
The cell suspension was transferred to 100-mm plastic culture
dishes (Corning), which were placed in an incubator at 37°C for
90 min. The myocytes remaining in suspension were then plated
onto new plastic culture dishes at a density of 5
×
10
5
cells/ml
for culturing. Cell viability at plating was assessed by trypan blue
exclusion. To test the purity of the myocytes, they were subjected
to immunocytochemical staining for expression of myocardial
sarcomeric actin. On the fourth day of culturing, the cells were
classified into various groups and incubated under normoxic
(20% O
2
) or hypoxic (5% O
2
, 2% O
2
, 1% O
2
) conditions. In
addition, cells cultured under conditions of 1% O
2
were given 5
μ
mol/l YC-1 to inhibit HIF-1
α
activity.
Hoechst 33258 DNA staining
Nuclear staining with Hoechst 33258 was assessed to detect
chromatin condensation or nuclear fragmentation, which are
characteristic of apoptosis. Cells cultured on glass slides were
fixed with 4% paraformaldehyde and stained with 1
µ
g/ml
Hoechst 33258 (Sigma) for 10 min at room temperature. The
cells were then washed three times with sterilised, distilled H
2
O.
Cells were counted and 200 were isolated and scored for the
incidence of apoptotic chromatin changes using a fluorescence
microscope (TE 300, Nikon). Three independent investigators
counted the cells.
Protein extraction and western blotting
Cells were washed and scraped from the dishes. Cellular total
protein was extracted by five packed-cell volumes of ice-cold
lysis buffer (containing 10 mM Tris-HCl, pH 7.8; 1.5 mM ethyl-
enediamine tetra-acetic acid (EDTA); 10 mMKCl; 0.5 mM dithi-
othreitol (DTT); 1 mM sodium orthovanadate; 2 mM levamisole;
0.5 mM benzamidine; and 0.05% Nonidet P-40) containing a
protease inhibitor cocktail (Sigma), and three rounds of sonica-
tion (5 s, 4°C). Protein concentrations were determined using the
Bio-Rad Bradford assay kit (Bio-Rad). Equal amounts of total
proteins were separated by sodium dodecyl sulfate-polyacryla-
mide gel electrophoresis (SDS-PAGE) and then transferred to
Immobilon-P membranes (Millipore). Membranes were blocked
with 5% non-fat milk at room temperature for one hour and then
incubated overnight at 4°C with primary antibodies, then incu-
bated using a secondary antibody and detected using the diami-
nobenzidine detection kit (DAB kit, Amersham Pharmacia).
The primary antibodies used were antibodies to HIF-1
α
(H-206, Santa Cruz), Bax (N20, Santa Cruz), Bad (C20, Santa
Cruz), Nip3 (C-18, Santa Cruz), and actin (Act40, Sigma). The
secondary antibody was donkey anti-goat IgG-HRP or goat
anti-rabbit IgG-HRP (Santa Cruz). For quantification purposes,
densitometric measurements were performed using the Quantity
One 1-D analysis software for Windows (Bio-Rad). The data
from the western blot analysis were expressed as relative
density/
β
-actin.
Data analysis
The results were expressed as mean
±
standard deviation (SD).
For multiple comparisons, results were analysed by analysis of
variance (ANOVA) and the least-significant difference
post-hoc
test was used to identify significant differences between the
individual cell groups;
p
<
0.05 was considered as statistically
significant. All statistical analyses were performed using the
SPSS 11.5 software.
Results
Effect of hypoxia
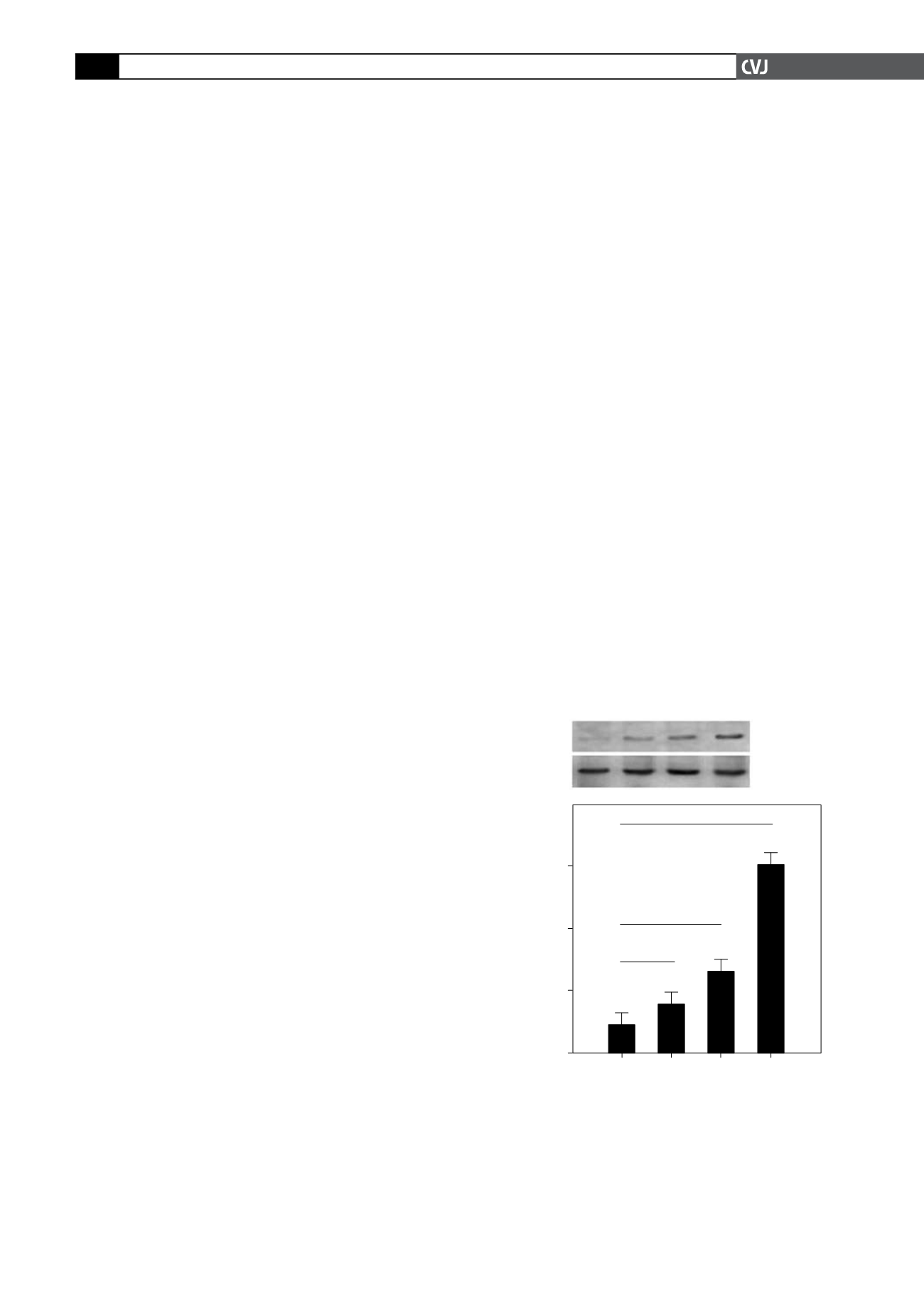
First, we investigated whether exposure to hypoxia would increase
the expression level of HIF-1
α
and the degree of apoptosis in
primary neonatal rat ventricular myocytes. Primary neonatal rat
ventricular myocytes on the fourth day of culture were incubated
under conditions of normoxia or different degrees of hypoxia. Our
data showed that under the normoxic condition, the level ofHIF-1
α
expression was low. As expected, the expression level of HIF-1
α
increased significantly (
p
<
0.05,
p
<
0.01; Fig. 1) in response
to hypoxia in a manner dependent on the degree of hypoxia.
The apoptotic rate in the ventricular myocytes cultured
under hypoxic conditions was significantly higher than that in
the controls; the increase in the apoptotic rate of the former
increased with the degree of hypoxia (apoptotic rate: 9
±
2%
in cells cultured under normoxic conditions; 26
±
5.4% in cells
cultured at 5% O
2
; 42
±
6.2% in cells cultured at 2% O
2
; and 62
±
5.4% in cells cultured at 1% O
2
(
p
<
0.01; Fig. 2).
Fig. 1. HIF-1
α
protein was induced by hypoxia in primary
neonatal rat ventricular myocytes in a degree-dependent
manner. HIF-1
α
protein expression in cells cultured for 24
hours under normoxic (20% O2) conditions and different
degrees of hypoxia (5% O
2
, 2% O
2
and 1% O
2
). (A) Total
proteins were subjected to immunoblotting analysis
with anti-HIF-1
α
or anti-
β
-actin. (B) Quantification of the
expression level of HIF-1
α
(*
p
<
0.05, #
p
<
0.01).
A
B
Oxygen concentration
20% 5% 2% 1%
HIF-1
a
120 kD
b
-actin
42 kD
Relative density
(HIF-1
a
/
b
-actin)
20% 5% 2% 1%
Oxygen concentration
0.60
0.40
0.20
0.00
*
#
#


