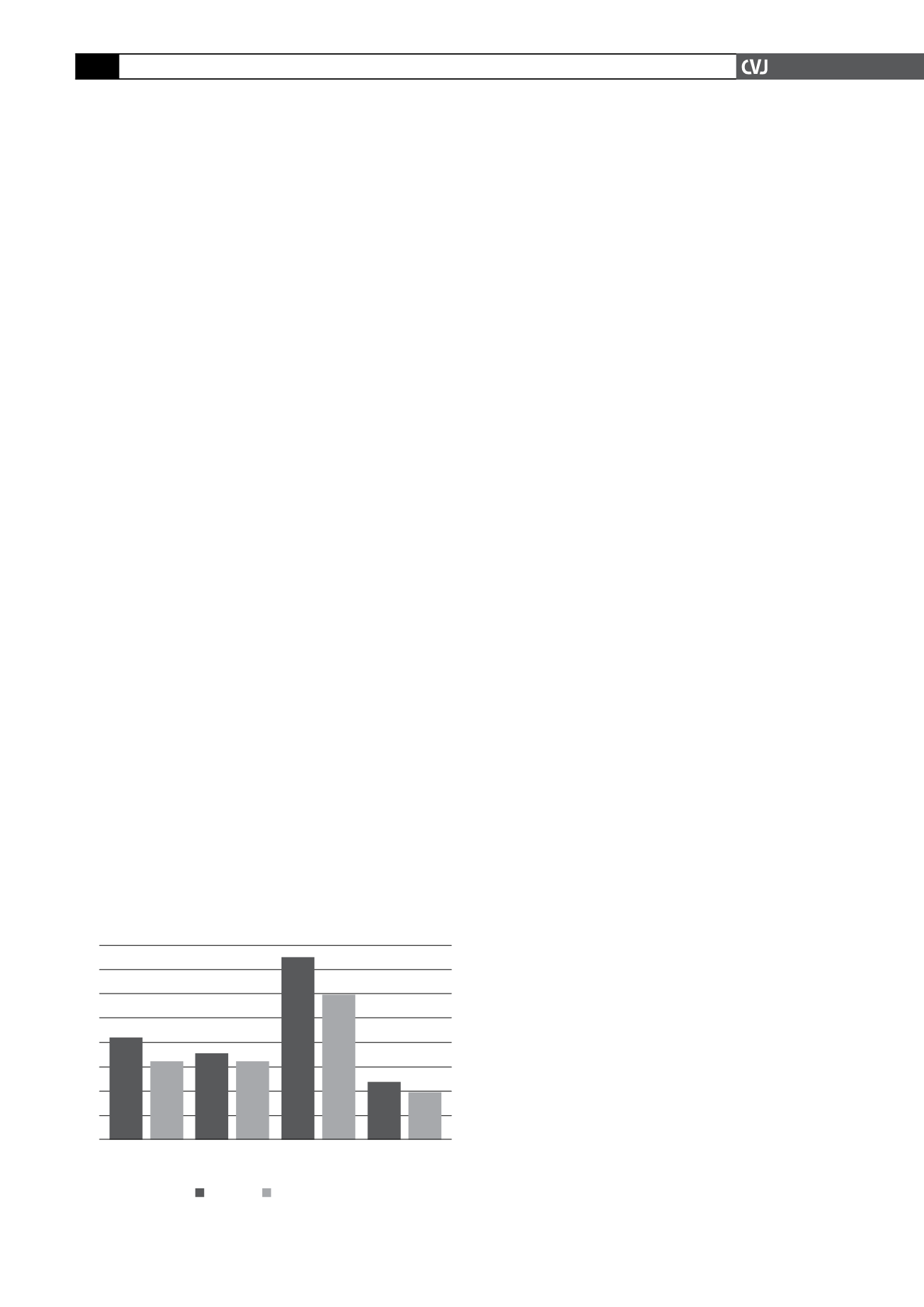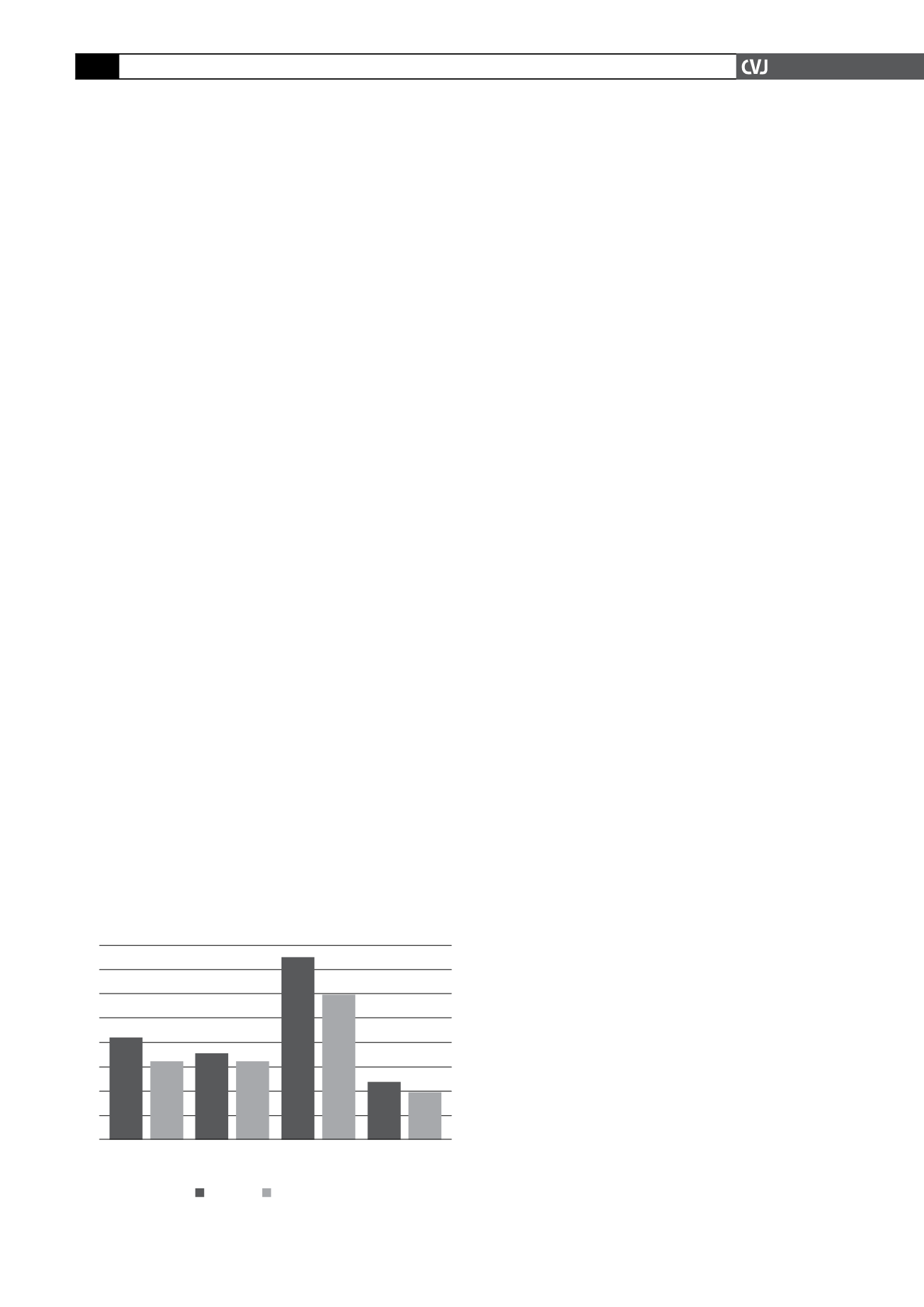
CARDIOVASCULAR JOURNAL OF AFRICA • Vol 21, No 1, January/February 2010
44
AFRICA
With such a large study as FIELD
4
now available, the poten-
tial of a pleiotropic effect associated with Lipanthyl will be
explored. Bearing these arguments in mind, the development of
economic evidence as part of the portfolio of evidence associated
with Lipanthyl is not only justified, but is critical to position the
molecule in the market window presenting itself at present.
In the FIELD study at five-year follow up, a similar propor-
tion of subjects in both groups (10–11%) had discontinued
therapy (FIELD study group: 2005).
1
However, those randomised
to placebo were more likely to require supplementary lipid-
lowering therapy (in this case, statin therapy) compared to the
fenofibrate group. There was no difference in the rate of starting
other lipid-lowering treatment between the groups in those with a
history of cardiac events (23 vs 14%) and those without a history
at baseline (16 vs 7%). However, for those with previous CVD,
the average rate was higher.
Despite a similar all-cause mortality in the FIELD study
1
(6.6% in the placebo group vs 7.3% in the fenofibrate group),
fenofibrate was associated with (see also Fig. 1):
11% risk reduction for a coronary event (
●●
p
=
0.16)
24% risk reduction for non-fatal MI (
●●
p
=
0.010)
19% risk increase for CHD mortality (
●●
p
=
0.22)
11% risk reduction for a cardiovascular disease event (
●●
p
=
0.035)
21% risk reduction for coronary revascularisation (
●●
p
=
0.003).
Although fenofibrate did not significantly reduce the risk of
the primary outcome (coronary events) relative to treatment
with placebo (standard treatment), it was associated with fewer
non-fatal myocardial infarctions and revascularisations in this
relatively high-risk group of patients.
Study aim
This analysis has developed an economic assessment of the
reduction of the risk associated with myocardial infarction,
stroke and the need for angiography and revascularisation in type
2 diabetes mellitus patients. This study therefore aimed to prove
that the effect of Lipanthyl in reducing the risk associated with
cardiovascular disease in subjects with type 2 diabetes melli-
tus constituted a cost-saving intervention. In order to test this
hypothesis, a cost–consequence analysis was followed up with a
cost–benefit analysis.
Methods
Study design
The study was a localisation of the Carrington and Stewart study
reported in the
International Journal of Cardiology
.
2
The meth-
odology used in their study was applied here as well, to ensure
integrity of the findings. This included the parameters used in
the study. The methodology used by Carrington and Stewart is
described comprehensively in their article, but for convenience,
it will be summarised here, indicating which adaptations were
required to localise the study.
This was a retrospective pharmaco-economic study based on
cost–consequence and cost–benefit analyses. The study popula-
tion consisted of patients suffering from type 2 diabetes mellitus,
as in the FIELD study.
1
Significance levels and the power of the
test have been derived from the FIELD study
The total treatment cost was determined for the Lipanthyl and
control groups. In the process of calculation, clinical effects and
resource utilisation were analysed and reported. The incidence of
all clinical events is reported as events per 1 000 person years.
The clinical effects that were considered included:
non-fatal myocardial infarctions (ICD10 codes I23 to I25)
●●
as in the Carrington article, it was assumed that all patients
●●
with a reported non-fatal MI underwent a coronary angiogram
(CPT codes 93543/5 and 93555)
stroke (ischaemic stroke: ICD10 codes I63–I64 and haemor-
●●
rhagic stroke: ICD10 codes I61–I62)
coronary revascularisation (limited to stents – both bare metal
●●
stents and drug-eluting stents were included at the rate in which
they occurred in the database of a local healthcare funder); the
fact that coronary artery bypass grafts were not included may
be considered to understate the event costs and would there-
fore constitute a conservative estimation of costs in this regard
non-coronary revascularisation.
●●
The resource effects considered consisted of:
hospitalisation costs
●●
pharmacotherapy
●●
professional fees.
●●
The discount rate used in this analysis was 10%, which is a
conservative estimate of the 2008 R153 government bond rate.
A private-sector funder perspective was used.
The active intervention used in the comparison was Lipanthyl
200 mg/day as per the FIELD study.
1
The control group in this
comparative study was placebo, which in this case refers to
standard treatment. The lipid-lowering therapy introduced in this
analysis was 10 mg of simvastatin at the average generic price
for the South African private sector. This can be considered to
be a conservative approach and adding any other lipid-lowering
agents would have only increased the difference in costs associ-
ated with the two comparators. All acquisition costs are reported
as retail price ex VAT.
Analysis
The analysis was based on the intention-to-treat population from
the FIELD study.
1
The time frame for the base case analysis has
been derived from the FIELD study and was five years or 60
months.
All the clinical outcomes were derived from the FIELD study.
One-way sensitivity analyses on risk reduction were done to
assess the robustness of clinical findings as well as assumptions
Fig. 1. Event rate per 1 000 person years at risk.
16
14
12
10
8
6
4
2
0
Placebo Fenofibrate
Non-fatal MI
Stroke
Coronary
revasculari-
sation
Other
revasculari-
sation