
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 28, No 6, November/December 2017
AFRICA
371
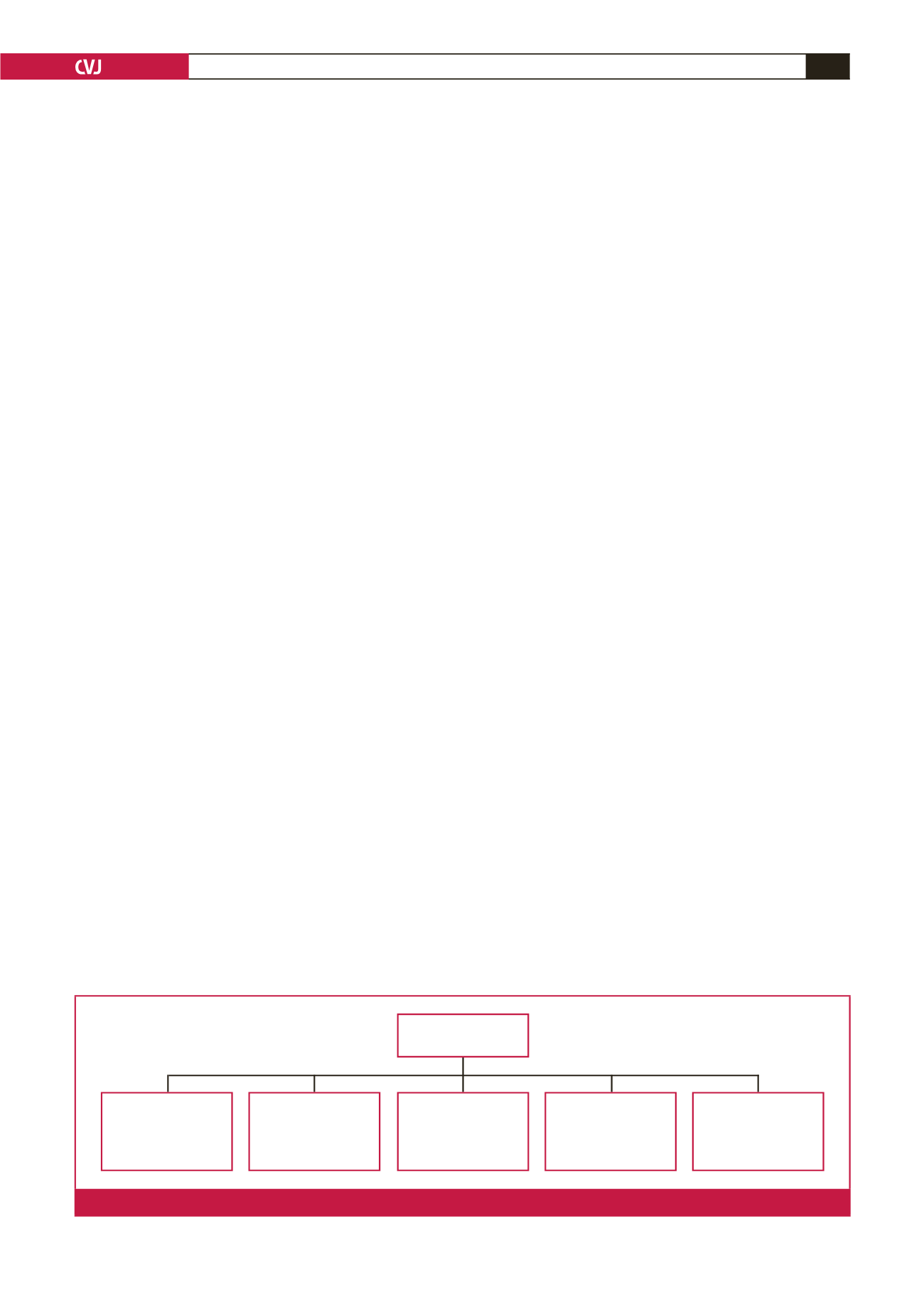
(PAH), venous, hypoxic, thromboembolic and miscellaneous
3
(Fig. 1). Overall, PH results from varying combinations of
increases in pulmonary vascular resistance, pulmonary blood
flow and pulmonary venous pressure. Sustained pressure
overload secondary to chronic PH leads to right ventricular (RV)
changes, including hypertrophy, dilatation and RV failure, which
are detectable using non-invasive tests such as electrocardiogram
(ECG), echocardiography, cardiac magnetic resonance or at best,
the gold standard but invasive right heart catheterisation (RHC).
Despite improvements in the understanding of PH and the
development of novel therapies, the condition is still diagnosed
at an advanced stage in a significant proportion of patients, due
to the paucity of symptoms in the early stages of the disease. This
has a negative impact on the quality of life and survival rate of
patients.
4
The American College of Cardiology/American Heart
Association
5
and the European Society of Cardiology/European
Respiratory Society
4
guidelines recommend the ECG as an initial
tool in diagnosing patients with suspected PH, based on studies
done predominantly in patients with PAH. However, these
guidelines consider ECG to be an inadequate tool for screening
and emphasise the advantage of Doppler echocardiography.
In sub-Saharan Africa (SSA) where chronic and endemic
precursors of PH, including chronic infectious diseases,
hypertensive heart disease, cardiomyopathy and rheumatic heart
disease are highly prevalent,
6
early diagnosis of PH is of particular
relevance. The high cost and low availability of, and need for
expertise in echocardiography limit its utility in this part of the
world and justify the interest in alternative tests such as ECG.
ECG abnormalities in patients with PH have been
predominantly described in other populations.
7-11
The
Pan-African Pulmonary Hypertension Cohort (PAPUCO) was
established to map out the epidemiology of PH in SSA. In
this sub-study, we aimed to assess the predictive value of an
affordable, widely available, objective and reproducible test such
as ECG to diagnose PH in resource-limited settings.
Methods
As previously described,
12
the PAPUCO study was a prospective,
registry-type cohort study of PH in Africa. The registry aimed
to recruit consecutive patients with newly diagnosed PH based
on clinical and echocardiographic criteria, who would be able or
likely to return for a six-month follow up, who were at least 18
years old (except for those in paediatric centres in Mozambique
and Nigeria), and who consented in writing to participate in the
registry.
Centre eligibility included availability of echocardiography,
training in assessing right heart function, experience in
diagnosing PH according to the WHO classification, experience
in clinical management of patients with right heart failure
(RHF), and resources to review patients at six-month follow up.
Participating centres were invited to join the registry at African
cardiac meetings and conferences.
12
The Heart of Soweto study
was a study of 387 urban South Africans of predominantly
African descent, determined to be heart disease free (using the
Minnesota code) following advanced cardiological assessment,
including echocardiography.
13
PH was diagnosed by specialist cardiologists using the
non-invasive definition of PH. The standard is a pathological
condition with an increase in mean pulmonary arterial pressure
(PAP) beyond 25 mmHg at rest, as assessed by RHC.
14
Because
RHC is seldom available in our setting, PH was diagnosed
in patients with a documented elevation in right ventricular
systolic pressure (RVSP) above 35 mmHg on transthoracic
echocardiography in the absence of pulmonary stenosis and
acute RHF, usually accompanied by shortness of breath, fatigue,
peripheral oedema and other cardiovascular symptoms, and
possibly ECG and chest X-ray changes in keeping with PH,
as per the European Society of Cardiology and European
Respiratory Society (ESC/ERS) guidelines on PH.
4
We searched ECGs from the PAPUCO registry to identify all
patients who had had bothDoppler echocardiography and 12-lead
ECG performed within 48 hours of their baseline inclusion. We
excluded all patients with pacemakers (due to inapplicability of
standard ECG criteria), poor-quality ECGs and those without
measurable RVSP. Controls were non-smokers and asymptomatic
subjects with normal Doppler echocardiography (and RVSP less
than 35 mmHg) who all underwent ECG recordings during their
baseline inclusion in the Heart of Soweto study.
15
This study
represents urban South African men and women, all free of any
heart disease and other major forms of cardiovascular disease.
All ECGs were reviewed and interpreted by two independent
clinical cardiologists who were blinded to the echocardiography
results. If consensus could not be reached, a third opinion (AD,
FT or KS) was requested. We electively studied pre-specified
ECG patterns classified into minor or major abnormalities, as
previously described in a large African cohort of heart disease-free
Africans.
13
Minor abnormalities included sinus tachycardia (
>
100
beats per min), minor T-wave changes (T-wave flattening) or early
repolarisation, definitive right ventricular hypertrophy (QRS axis
≥
+
100° or R/S ratio in V1
≥
1, or R in V1
>
7 mm or a combination
of a right bundle branch block and QRS axis
≥ +
100°).
Pulmonary
hypertension (PH)
Group 3: PH due to
lung diseases and/or
hypoxaemia
e.g. chronic obstructive
pulmonary disease
Group 4: Chronic
thromboembolic CPH
e.g. chronic pulmonary
embolism
Group 5: PH with
unclear or multifactorial
mechanisms
e.g. endomyocardial
fibrosis
Group 2: PH due to left
heart disease
e.g. mitral stenosis
due to rheumatic heart
disease
Group 1: Pulmonary arte-
rial hypertension (PAH)
e.g. human immuno-
deficiency virus-
associated PAH
Fig. 1.
World Health Organisation classification for PH adapted from the 5th World Symposium on Pulmonary Hypertension.
3

















