CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 8, September 2013
AFRICA
325
to ischaemia–reperfusion were shown to produce IL-6 in several
experimental models.
19
In order to improve clinical outcomes
in open-heart surgery with CPB, oxidative stress should be
prevented by decreasing reperfusion injury and inflammation.
Despite improvements in surgical techniques, inflammation
continues to be an important problem during these procedures.
Wan
et al.
have shown that CPB may induce complement
or leukocyte activation, endotoxin release, the expression of
adhesion molecules, and the release of inflammatory mediators.
2
Moreover, the heart itself is a major source of inflammatory
mediators and oxygen-derived free radical species that are likely
to contribute to the impairment of cardiac pump function.
20
LA administration has been shown to be advantageous
in a number of oxidative stress models such as ischaemia–
reperfusion injury, diabetes and cataract formation. In the
present study, the beneficial effects of LA were manifested by
statistically significant decreases in plasma IL-6, IL-8, C3, C4,
CRP and haptoglobulin levels. LA decreased levels of IL-6 in the
P2, P3 and P4 periods and decreased levels of IL-8 in the P2 and
P3 periods. When compared with the controls, LA significantly
decreased IL-6 and IL-8 synthesis in a time-depended manner.
LA may act as extra- and intracellular redox signaling couples
and a powerful free radical scavenger, suggesting that LA has a
possible therapeutic agent in surgeries where ECC is used.
21
Similarly, Steinberg
et al
. reported that IL-6 levels increased
after protamine administration and reached a maximum level
three hours after bypass. At 24 hours after bypass, IL-6 levels
remained above the levels measured at initiation. Our results
showed that IL-6 levels in both groups were above the P1 levels
at P2, P3 and P4. It has been previously reported that LA was
able to increase intracellular GSH levels, which is the most
abundant cellular antioxidant, by acting as a buffer system for the
thiol redox state.
6,22
Nowadays, there is strong evidence that LA
is one of the modifiers of critical protein thiolates and therefore
may influence the pathways of thiol redox state.
23,24
Furthermore, GSH is implicated in the recycling of
antioxidant vitamins such as vitamins E and C, which participate
in modulating the activity of superoxide dismutase enzyme.
Currently, there is mounting evidence that LA increases the
levels of the cellular antioxidant enzyme GSH by acting as a
transcriptional inducer of genes governing GSH synthesis.
25
Glutathione peroxidase (GSH-Px) and GSH act as antioxidant
molecules and have protective effects against reactive oxygen-
derived molecule-triggered degeneration. GSH is one of the
most important antioxidant molecules for removing lipid
hydroperoxides and hydrogen peroxide.
26,27
It is one of the
precursors for catalysing hydrogen peroxide to water.
The two major sources of intracellular ROS production are
mitochondria and the plasma membrane-bound multicomponent
enzyme complex NADPH oxidase.
28
Kagan
et al
. also mentioned
that LA interacts with NADPH or NADH-dependent electron
transport chains to recycle vitamin E.
29
LA is well known as
an inhibitor of nuclear factor (NF-
kβ
).
7
LA decreases TNF-
α
-
induced NF-
kβ
activation and the expression of adhesion
molecules in endothelial cells, and thereby it may reduce the
inflammatory response.
25,30,31
In inflammatory diseases, membrane damage appears
frequently in cells that incite lipid peroxidation and disturbances
in membrane structure.
32
When lipid peroxides aggregate to a
certain level, they leak from the cells into the blood and increase
lipid peroxidation in the blood plasma. Melek
et al
. determined
increased levels of CRP during ECC.
33
CRP is one of the
indicators of inflammation activated by cytokines in the liver.
In our study, the levels of CRP increased in the P4 period,
following IL-6 and IL-8 increase. This demonstrated that CRP
activation is depended on LA synthesis. These changes are also
considered to be a consequence of imbalance between oxidant
products and antioxidant defense mechanisms. This kind of
systemic inflammatory response to CPB has the potential of
bringing about clinical and cellular disorders.
Maulik
et al
.
34
demonstrated that oxidative stress triggered
apoptosis in re-perfused hearts in swine. This relatively unknown
anti-inflammatory effect of LA may contribute to the inhibition
of ECC-induced inflammation
in vivo
and reduce ECC-related
adverse effects.
Conclusion
ECC is an important innovation in CPB, but its safety is
not guaranteed due to the inflammatory reaction generated
by ECC.
35,36
Systemic inflammatory reactions cause serious
complications, which may affect postoperative mortality
in cardiac surgery patients. Therefore the originality of our
findings and the potential benefits of using LA during cardiac
surgery could be useful.
37,38
Future research will be directed at
finding the unique pharmacological and biological agents or
their combinations, which may effectively reduce ECC-caused
inflammatory responses. An appropriate strategy to inhibit
ECC-triggered inflammation could be beneficial for patients
undergoing cardiac surgery using ECC.
References
1.
Schmid E, Krajewski S, Bachmann D,
et al
. The volatile anesthetic
sevoflurane inhibits activation of neutrophil granulocytes during
simulated extracorporeal circulation.
Int Immunopharmacol
2012;
14
:
202–208.
2.
Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopul-
monary bypass: Mechanisms involved and possible therapeutic strate-
gies.
Chest
1997;
112
: 676–692.
3.
Edmunds LH,Jr. Inflammatory response to cardiopulmonary bypass.
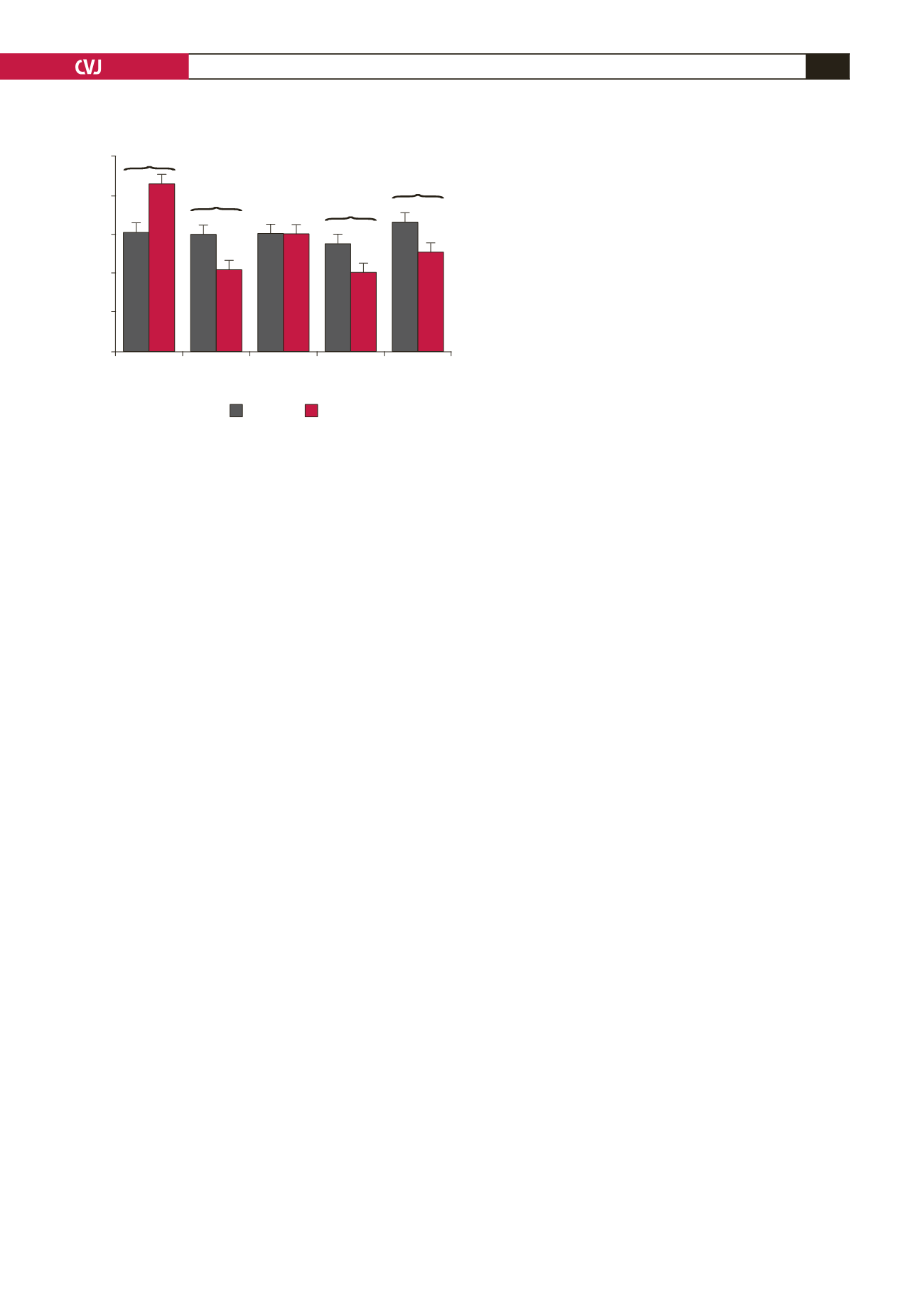
Fig. 5. Anti-streptolysin (ASO) levels in serum were
analysed using a turbidimetric method. *
p
<
0.05 indi-
cates statistical significance versus the respective base-
line value in each group.
#
p
<
0.05 indicates statistical
significance between the two groups at each time point.
250
200
150
100
50
0
P1
P2
P3
P4
P5
Time periods
ASO (IU/ml)
Control
LA
*
*
*
*
#
#
*
*
#
#


