CARDIOVASCULAR JOURNAL OF AFRICA • Volume 25, No 3, May/June 2014
AFRICA
119
Methods
All experimental procedures were performed with the approval
of the Faculty of Health Sciences Animal Ethics Committee,
University of Cape Town. All protocols were carried out in
compliance with the European Convention for the Protection of
Vertebrate Animals used for Experimental and other Scientific
Purposes (Council of Europe No 123, Strasbourg 1985).
Male Wistar rats (250–300 g,
n
=
56), wild-type and
cardiomyocyte-specific STAT-3 knockout mice (14–16 weeks,
n
=
31) were bred and obtained from the University of Cape Town
Animal Unit as previously described.
6
Isolated STAT-3 knockout heart model
Cardiomyocyte-specific STAT-3 knockout mice (STAT-3 KO)
and wild-type littermate control mice were anaesthetised
(sodium pentobarbitone, 60 mg/kg i.p.) and heparinised (25 IU
i.p.). Once an adequate level of anaesthesia was achieved, the
chest was opened, the heart was rapidly removed and placed in
ice cold (4
o
C) modified Krebs-Henseleit buffer, and the aorta
was cannulated.
The hearts were then perfused with Krebs-Henseleit buffer
using the Langendorff system as previously described.
25
A
minimum of 1.5 ml/min and maximum of 5.0 ml/min of
coronary flow rate, heart rate between 460 and 600 beats per
minute (bpm) and developed force
≥
4 g was deemed acceptable.
No haemodynamic data were collected during the protocol.
After a 20-minute stabilisationperiod, the heartswere subjected
to 35 minutes of global ischaemia followed by 45 minutes of
reperfusion. Hearts were pre-treated with S1P (10 nmol/l in
DMSO) for seven minutes, followed by a 10-minute washout
period before global ischaemia, as previously described.
14
At the
end of each experimental protocol, the infarct size was assessed
by triphenyltetrazolium chloride (TTC) staining. The infarct size
was determined with planimetry.
25
Isolated rat heart model
The rats were anaesthetised with sodium pentobarbital (50 mg/
kg i.p.) and heparinised (500 IU i.v.). The hearts were rapidly
excised and perfused retrogradely by the Langendorff technique,
as previously described.
25
Rat hearts that did not comply with
the following criteria were excluded: (1) left ventricular pressure
greater than 80 mmHg, (2) coronary flow rate at a minimum of
8 ml/min and maximum of 16 ml/min, (3) heart rate at a
minimum of 240 bpm and maximum of 400 bpm.
After 30 minutes of stabilisation, all hearts were subjected
to 30 minutes of regional standard ischaemia by occlusion of
the left coronary artery and 120 minutes of reperfusion, as
previously described.
25
Hearts were pre-treated with S1P (10
nmol/l in DMSO) for seven minutes followed by a 10-minute
washout period before the standard ischaemia. In half of the rats,
the JAK-STAT-3 inhibitor, AG490 (100 nmol/l),
26
was given for
15 minutes: three minutes before, seven minutes concomitantly
with S1P (S1P + AG490 group) and five minutes after perfusion
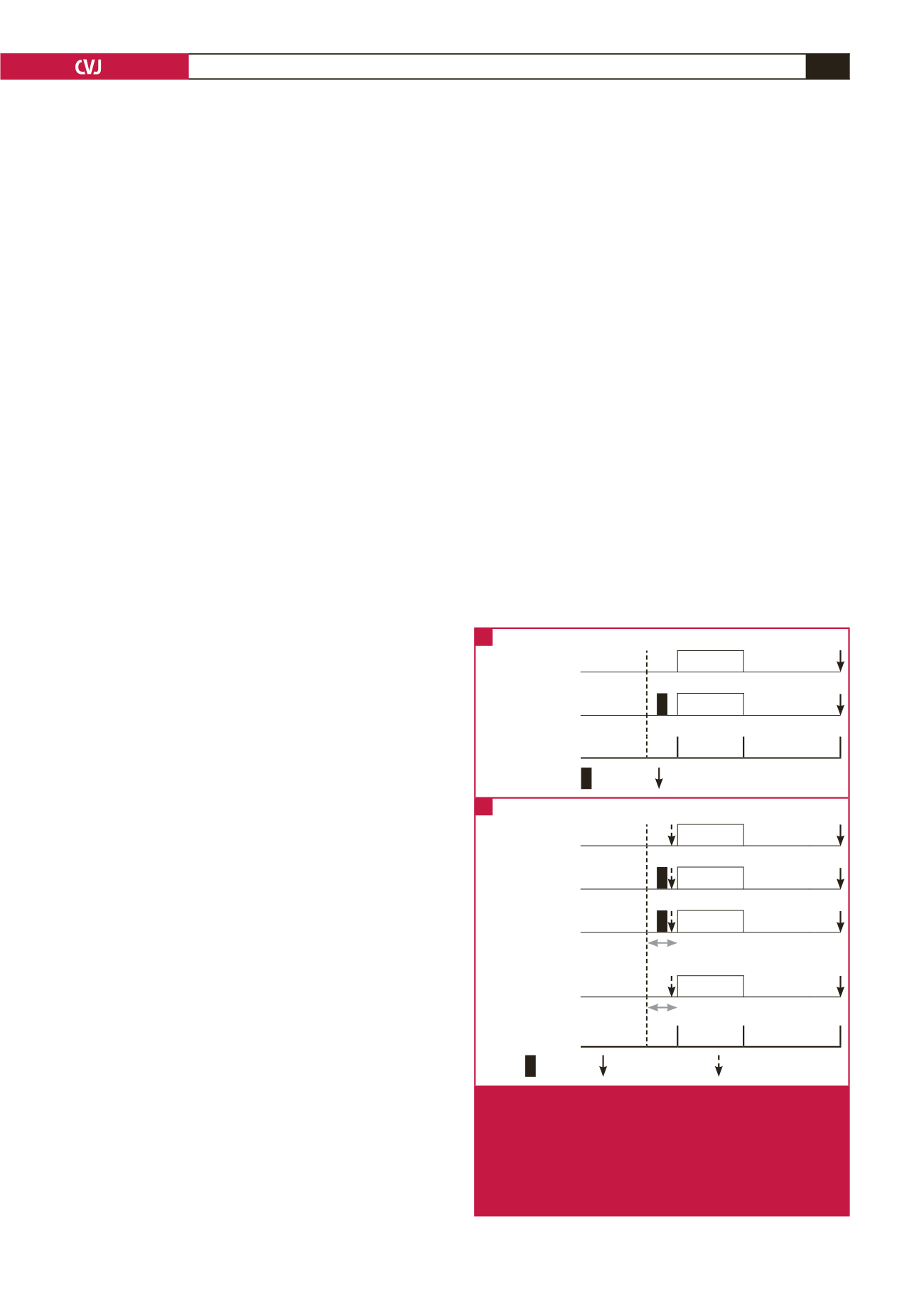
with S1P (Fig. 1).
Haemodynamic parameters were assessed throughout the
experiment and included heart rate, left ventricular developed
pressure (LVDP) and coronary flow. Haemodynamic variables
were statistically tested for intergroup and intragroup variation.
For the measurement of infarct size, the coronary artery was
re-occluded at the end of the reperfusion period and a solution
of 2.5% Evans blue was perfused to delineate the area at risk
(AAR).
The hearts were then frozen and cut into slices, and incubated
in sodium phosphate buffer containing 1% w/v TTC for 15
minutes to visualise the unstained infarct region. The infarct size
and AAR were determined with planimetry and the infarct size
was expressed as a percentage of the AAR.
Preparation of hearts for Western blots
In the isolated rat hearts, the ventricular tissue from control and
S1P pre-treated hearts was excised before the regional ischaemic
insult (seven minutes after S1P treatment), freeze clamped using
Wollenberger tongs in liquid nitrogen and stored at –80°C. The
frozen hearts were wrapped in aluminium foil and pulverised
under liquid nitrogen before being transferred to tubes for
storage.
For extraction of nuclear and cytosolic protein, pieces of the
left ventricle were homogenised twice by Polytron using the
homogenisation buffer described by Williams and Ford.
27
The
suspension was then centrifuged at 10 000
g
(12 000 rpm) for five
minutes at 4°C. The supernatant containing the cytosolic fraction
was collected and transferred into a fresh tube. The pelleted
fraction was resuspended in the same homogenisation buffer
supplemented with 1% Triton X-100, as described previously.
27
Control
Stabilsation
Ischaemia Reperfusion
S1P preconditioning Stabilsation
Ischaemia Reperfusion
20 min
35 min
45 min
S1P
Infarct size
Control
Stabilsation
Ischaemia Reperfusion
S1P preconditioning Stabilsation
Ischaemia Reperfusion
S1P + AG490
Stabilsation
Ischaemia Reperfusion
AG490
AG490
Stabilsation
Ischaemia Reperfusion
AG490
30 min
30 min
120 min
S1P
Infarct size
Western blot
7’
7’
7’
7’
Fig. 1.
Preconditioning protocols. (A) Schematic diagram of
isolated mouse hearts undergoing a precondition-
ing protocol with and without S1P pre-treatment. (B)
Schematic diagram of isolated mouse hearts under-
going a preconditioning protocol with and without S1P
pre-treatment. These protocols were repeated in the
presence of the STAT-3 inhibitor AG490.
A
B


