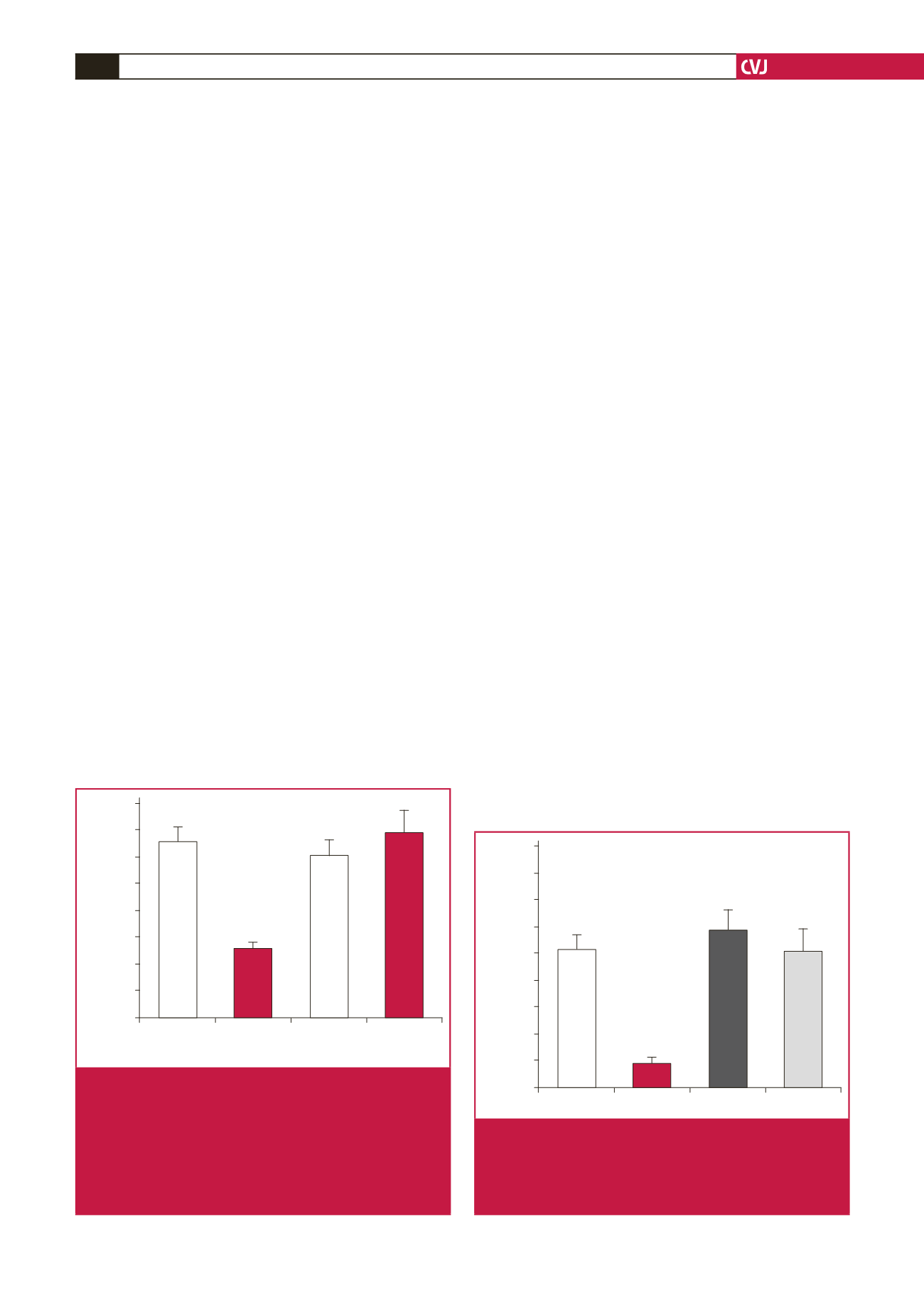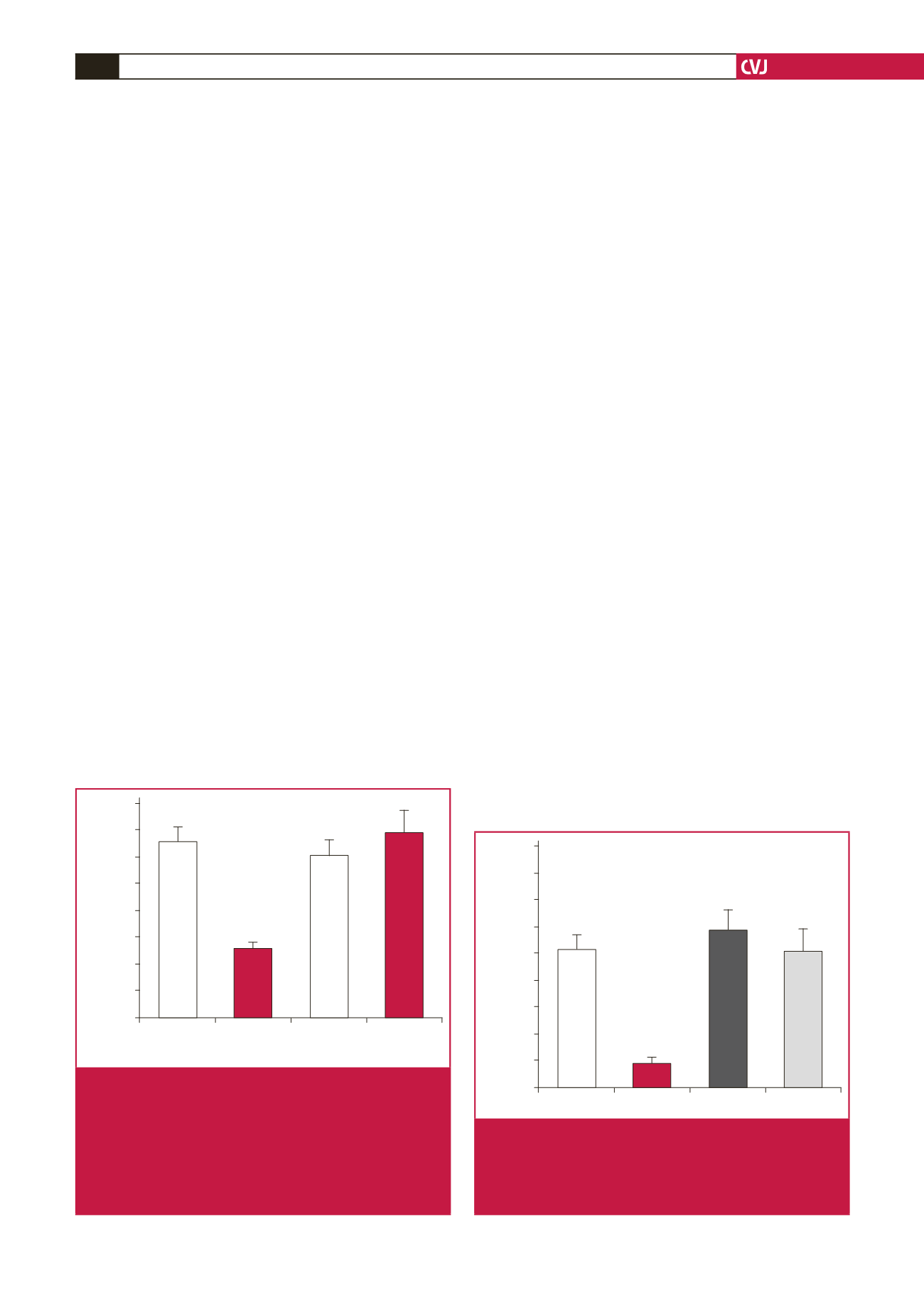
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 25, No 3, May/June 2014
120
AFRICA
After centrifugation for 30 minutes at 10 000
g
(12 000 rpm)
at 4°C, the supernatant containing the nuclear fraction was
carefully removed and transferred to a clean tube.
For extraction of mitochondrial and cytosolic protein, the
frozen rat hearts were finely minced with scissors in a lysis
buffer, as described by Lewin
et al
.,
28
and then transferred to a
Dounce homogeniser. After homogenisation, the suspension was
centrifuged at 600
g
for five minutes at 4°C. The supernatant
was transferred to a fresh micro-centrifuge tube and centrifuged
at 10 300
g
(11 500 rpm) for 10 minutes. The supernatant is now
the cytosolic fraction and the pellet the mitochondrial fraction.
The pellet was resuspended in 40 µl incubation buffer (250
mM sucrose, 25 mM Tris, 8.5 mM KH
2
PO
4
). The proteins
were quantitated and an equal volume low-ionic strength
sample buffer [10 % sodium dodecyl sulphate (SDS), glycerol,
mercaptoethanol, Tris (pH 6.8), bromophenol blue) was added
to each sample.
Western blot analysis
Phosphorylated and total STAT-3 levels were analysed by SDS
polyacrylamide gel electrophoresis with antibodies from Cell
Signalling Technology. Proteins were revealed with enhanced
chemiluminescence (ECL) Western blotting detection reagents
(Amersham, UK) and the images were captured electronically
using a GeneGnome HR (Syngene Bioimaging, UK).
Levels of phosphorylated and total STAT-3 were determined in
the same samples and under the same conditions but on separate
membranes. Equal loading was verified with
β
-actin staining
for the nuclear and cytoplasmic fractions and voltage-dependent
anion channel (VDAC) for the mitochondrial fractions. Levels
of phosphorylated proteins were normalised to their total protein
levels.
Relative densitometry was determined using Quantity One
software (Biorad). The cytoplasmic fraction analysed in these
experiments came from a different group of hearts, however all
hearts came from the same strain of rat of the same age and they
were treated identically.
Statistical analysis
Data are presented as mean
±
SEM. Comparisons between
multiple groups were performed by one-way ANOVA followed
by the Dunnet’s
post hoc
test (Graph Pad Instat). A value of
p
<
0.05 was considered statistically significant.
Results
S1P-induced preconditioning was inhibited in the
STAT-3 knockout mice
Control hearts subjected to 35 minutes of global ischaemia
and 45 minutes of reperfusion had an infarct size of 33
±
3%.
Pre-treatment with S1P (10 nmol/l) resulted in a significant
reduction in the infarct size to 13
±
1% (Fig. 2) (
p
<
0.05 vs
wild-type control hearts). Ischaemic control hearts from STAT-3
knockout mice had an infarct size of 30
±
3 %. The infarct-
sparing effect observed with S1P pre-treatment in the wild-type
hearts was absent in the knockout hearts (35
±
4%,
p
=
ns vs
control hearts) (Fig 2).
Of note, the present experimentswere conducted concomitantly
with our other experiments exploring the cardioprotective effect
of S1P as a postconditioning agent. The infarct size for the
control groups only [in both wild-type (
n
=
10) and knockout
animals (
n
=
8)] contributed to data already reported.
24
Inhibition of STAT-3 activation abrogated
protection by S1P-induced preconditioning
In the isolated rat heart model, the control hearts subjected to a
regional ischaemia–reperfusion insult had an infarct size of 26
±
8%. Pre-treatment with S1P (10 nmol/l) (Fig. 3) reduced the
infarct size (5
±
3% vs ischaemic control,
p
<
0.01,
n
=
6).
40
35
30
25
20
15
10
5
0
CTL
S1P
CTL
S1P
WT
KO
Infarct size (%)
*
Fig. 2.
The cardioprotective effect of S1P was abolished
in cardiomyocyte-specific STAT-3 knockout mice
subjected to ischaemia–reperfusion. In isolated
hearts from cardiac-specific STAT-3- knockout mice,
S1P failed to protect against an ischaemia–reperfu-
sion insult. (
n
≥
6 for all groups, *
p
<
0.05 vs wild-type
control). WT
=
wild type, KO
=
knockout. STAT-3
=
signal transducer and activator of transcription-3.
45
40
35
30
25
20
15
10
5
0
CTL
S1P S1P + AG AG
Infarct size (%)
*
Fig. 3.
S1P conferred protection via STAT-3 in the
Langendorff-perfused rat heart. Co-incubation of the
STAT-3 inhibitor AG490 (100 nmol/l) with S1P abol-
ished the infarct-sparing effect of S1P in isolated rat
hearts [
n
> 6 per group, *
p
<
0.01 vs control (CTL)].