
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 29, No 2, March/April 2018
100
AFRICA
clinical management, staff training, and on-site resources. The
majority of questions (34/45) followed a multiple-choice format
to quantify the challenges and opportunities encountered by
investigators during the study.
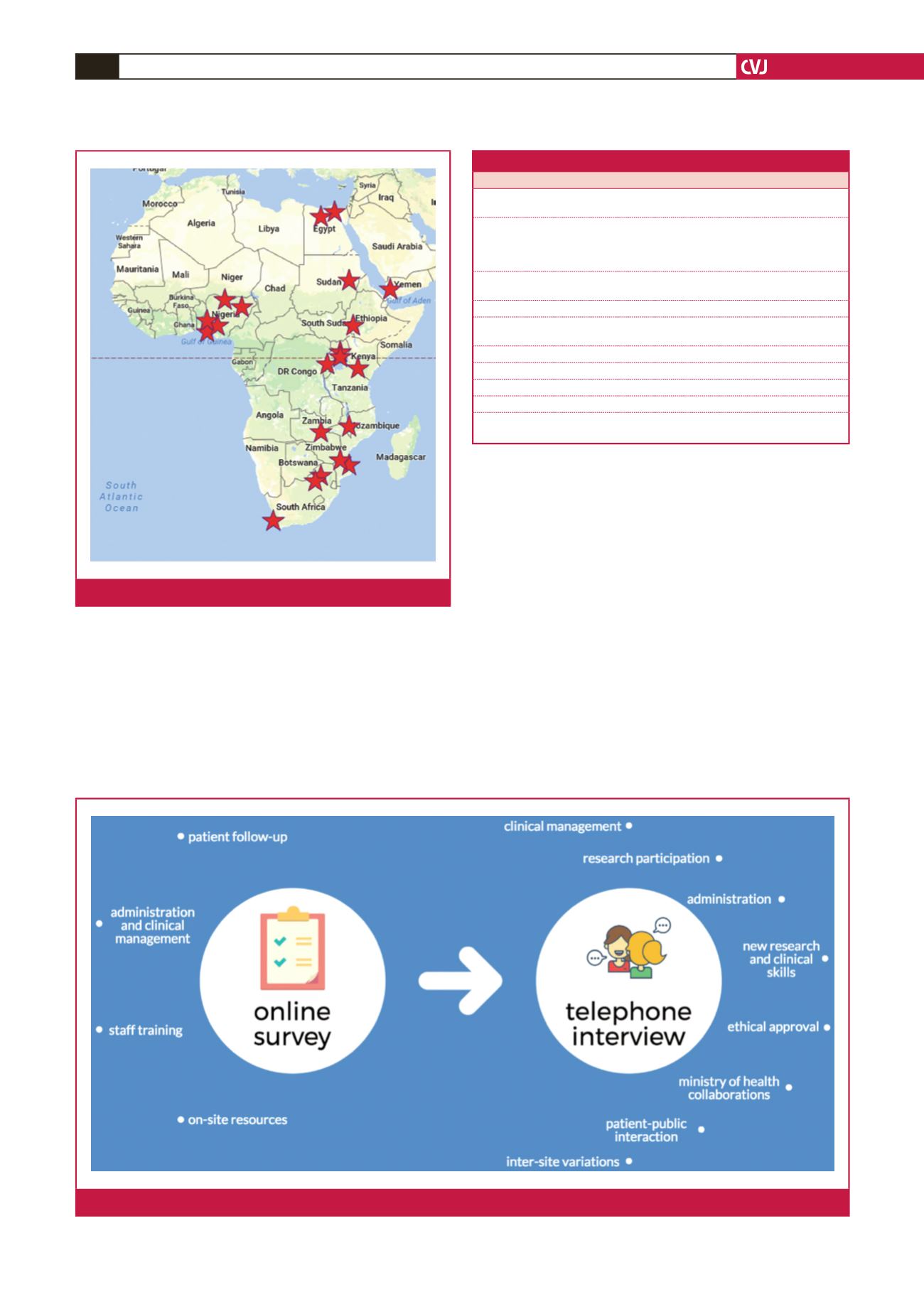
Thirty participants from 22 sites completed the survey (Fig.
1). Most survey respondents (19/30) also participated in a follow-
up telephonic interview (conducted by Skype, telephone or
WhatsApp call) comprising 10 qualitative questions concerning
their experiences during the study (Table 2). The telephonic
questionnaire focused on clinical management, research
participation, administration, research and clinical skills,
ministry of health collaborations, patient–public interactions,
inter-site variations and ethics approvals (Fig. 2).
Results
Online survey
Patient follow up: all respondents (30/30) experienced significant
difficulties with the follow up of their RHD patients. Participants
identified invalid telephone numbers (24/30), long distances
(24/30), medical costs to patients (20/30) and language barriers
(7/30) as common problems (Fig. 3). Other barriers included
late ethics committee approvals, lack of study funding, lack of
support from other on-site staff and difficulty in tracing patients’
addresses.
Fig. 1.
Participants from 22 sites completed the survey.
Table 2. Challenges and opportunities: the REMEDY study
Telephone interview questions
1. For many of the REMEDY sites, it was their first time participating in a multi-centre
research project. Was this the case for your site?
2. Some participants have said that REMEDY had an impact on the clinical, research,
academic and administrative aspects of their sites. For example, some reported
that it changed the way in which they ran clinics. What impact did REMEDY have
on your site?
3. Were you able to obtain additional resources by the fact that you were in the
REMEDY study?
4. Did REMEDY have any impact on your relationship with the Ministry of Health?
5. Some members of staff have said that they acquired some skills as a result of the
REMEDY study. Was this the case at your site?
6. Have you/they used these skills in other contexts?
7. Would you have liked an opportunity to learn anything else during the study?
8. What was your experience with ethics and institutional approval processes?
9. What, if any, impact has REMEDY had on your RHD patients?
10. Is there anything else that you would like to tell me that REMEDY did or did not do
for your site?
Fig. 2.
Information-gathering methods and their components.

















