
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 29, No 2, March/April 2018
AFRICA
103
echocardiographic findings, 9/19 stated that it had no impact and
5/19 did not comment on its effect.
New research and clinical skills: approximately two-thirds
of respondents (14/19) acquired research skills as a result of
REMEDY, such as protocol preparation, data management and
creation of case report forms, while 8/19 acquired new clinical
skills, such as improved interpretation of echocardiograms. The
vast majority (16/19) used these skills subsequently in other
contexts. Moreover, 14/19 remarked that they would have valued
the opportunity to learn further skills during the study. One
researcher suggested that their site would have benefitted from an
introductory research course to familiarise the research team with
the chronology of the study, required steps and study timelines.
Other suggestions included increased site-initiation training,
on-site visits to ensure quality control, statistical analysis courses,
increased guidance on how to perform echocardiography, and
more training in anticoagulant management.
Ministry of Health collaborations: for 11/19 researchers,
REMEDY had an impact on their site’s relationship with the
Ministry of Health. One investigator shared their site’s data with
the national Ministry of Health in order to procure assistance in
drawing up guidelines for the detection and prevention of RHD.
At a different site, the findings of REMEDY led to the creation
of a national RHD registry and investment in echocardiography
machines by the local Ministry of Health. Another site used
REMEDY results to collaborate with the Ministry of Health in
securing approval for RHD screening in schools.
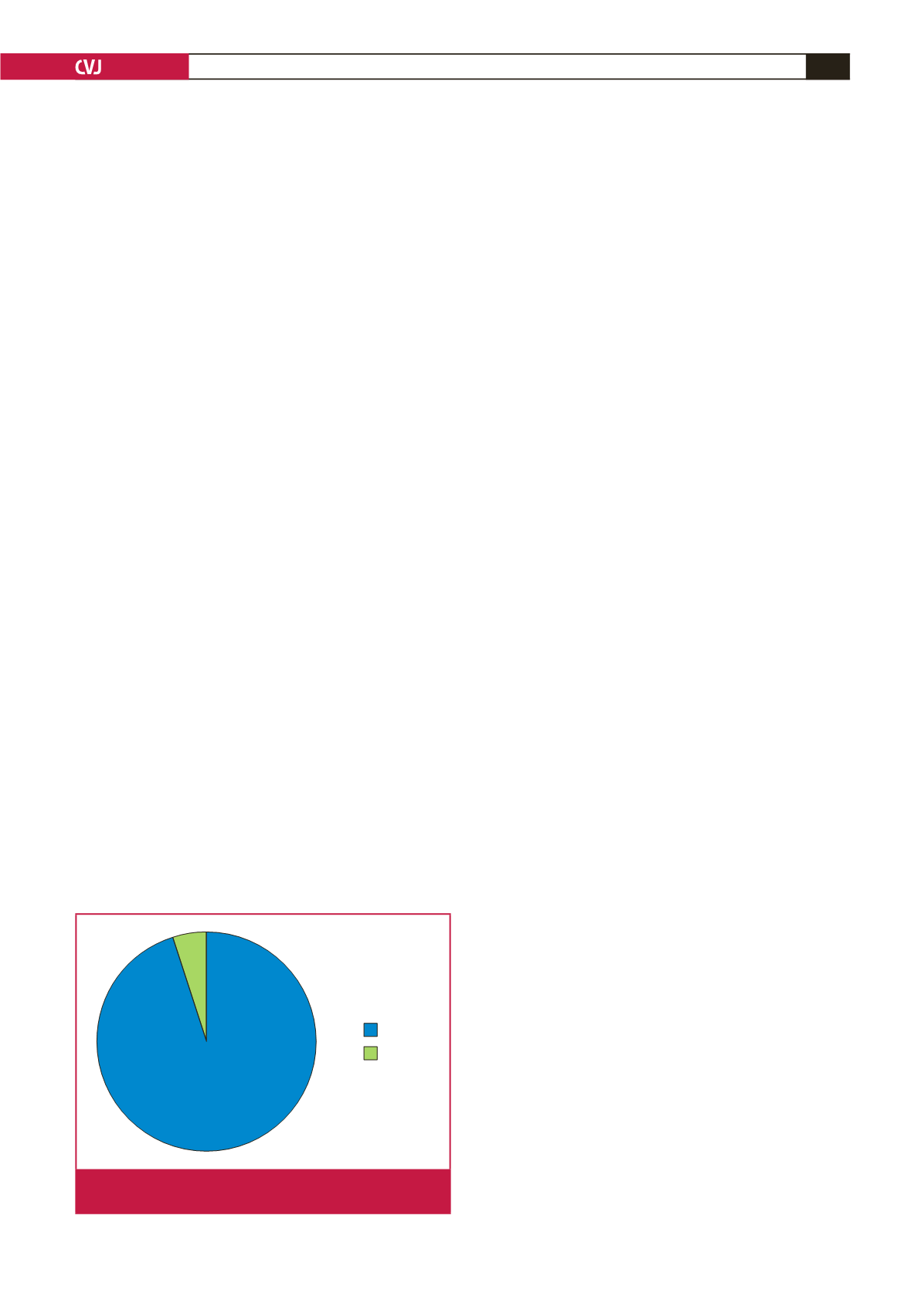
Patient–public interaction: almost all participants (18/19)
responded positively when asked whether participation in
REMEDY had an impact on interaction with their RHD
patients (Fig. 9). For example, investigators at the Groote Schuur
and Red Cross War Memorial Children’s Hospitals (Cape Town)
presented the outcomes of REMEDY to their RHD patients
in 2014. The hospital now hosts an annual event for its RHD
patients, which aims to empower patients by improving their
understanding of the disease and compliance with treatment.
Additionally, a patient community advisory board has been
established as a community liaison between patients and clinical
researchers. Similarly, the Uganda Heart Institute established a
patient support group in which long-term RHD patients support
clinicians in counselling newly diagnosed patients. Such groups
build patient confidence and empower them to manage their
disease.
Inter-site variations: Although the telephone findings were
largely consistent across study sites, wide variation in previous
exposure to multi-centre research, patient volume, and access
to healthcare resources provided a unique set of challenges and
opportunities at each centre. For example, 11/16 respondents
who had previously participated in multi-centre research (and
9/11 who had already conducted specific cardiology research)
employed pre-existing administrative, human and material
resources during REMEDY.
Furthermore, on-site support for REMEDY varied greatly
from one site to the next, with team sizes ranging from 20
members of staff to one principal investigator acting alone.
Those with limited support found the study taxing on their time
and resources and expressed that REMEDY had limited impact
on their site as a result. Several methods were employed by
investigators to resolve this challenge. For example, 8/19 sought
additional resources from non-governmental organisations, other
hospitals and pharmaceutical companies. One site established
a research committee with the intent of employing full-time
administrative staff to relieve pressure experienced by clinicians
in the course of research activities.
Ethical approval: given that REMEDY is a prospective,
non-interventional registry, 13/19 sites experienced little difficulty
in obtaining ethical clearance. Those that experienced difficulties
were unable to recruit large numbers of patients for the study,
demonstrating the importance of early application for ethical
and institutional approval.
Principal investigator responses
The principal investigators of REMEDYwere asked an additional
question about unexpected challenges during the course of the
study. A common problem was the variation in availability
of patient identifiers (such as date of birth, thumbprints and
formal addresses) across sites. Similarly, clinical records for each
patient’s history of stroke, HIV and contraceptive use differed
across sites, leading to underestimation of their incidence.
Political situations also impacted on the study’s progress.
Halfway through the study, South Sudan became an independent
nation, resulting in a 30% loss of Sudanese patients from the
registry. Furthermore, the civil war in Yemen and the Arab
Spring unrest in Cairo significantly hindered follow up of
REMEDY patients in these countries.
Both principal investigators therefore stressed the importance
of start-up and progress meetings in the course of a multi-
centre research project. Although challenging to implement,
monitoring and evaluation strategies allow investigators to
identify and address challenges encountered by individual sites.
For example, a monitoring visit to Zambia allowed the Project
Coordination Office to update REMEDY patient files on site,
while visits to other sites resulted in grants for fax machines,
cellphone data and laptop computers to enable the transmission
of REMEDY data to Cape Town.
Discussion
The impact of REMEDY on everyday practice in a variety
of geographical and clinical settings demonstrates that RHD
95%
5%
Impact
No impact
Fig. 9.
Telephone interview: impact of REMEDY on patient
behaviour.

















