
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 29, No 2, March/April 2018
AFRICA
125
disorders.
53
If clinically proven mtDNA mutations can directly
lead to cardiac dysfunction, is it plausible to think that other
mtDNA variants, such as population variants of mildly
deleterious effect, might also lead to or alter severity/penetrance
of complex cardiovascular disease phenotypes.
From the substantial supportive evidence of mitochondrial
involvement in cardiovascular disease, it is therefore evident
that genetic investigations on the aetiology of CVD should
include consideration of mtDNA variations. In the following
sections, we present a number of approaches (plus findings from
such investigations) on how mtDNA variation is investigated/
associates in/with disease, with a specific focus on the approaches
more likely to show its putative contribution to the risk of CVD
development.
Current approaches used for investigating
mtDNA involvement in disease
Mitochondrial DNA copy number
mtDNA copy number can be used as an indicative marker
of mitochondrial biogenesis, which is thought to increase in
response to increased energy demands, such as exercise, but also
as a compensatory method for mitochondrial dysfunction.
89
On the other hand, mtDNA copy number has been shown to
decrease with aging,
90
and has been significantly correlated
with late-onset diseases, such as Parkinson’s disease.
91,92
As
mentioned above, cell-free circulating mtDNA may also act as
an inflammatory agent that contributes to CVDs.
33
Altered mtDNA copy number measured in peripheral
blood cells have been shown to be associated with different
complications of diabetes (diabetic retinopathy and diabetic
nephropathy).
93,94
Also, an association between telomere length
and mtDNA copy number suggests a co-regulatory mechanism
for these two parameters, both of which are implicated in aging.
95
mtDNA depletion and impaired mitochondrial biogenesis have
been shown to be a constant factor in the early stages of heart
failure
96,97
and other diseases thought to be related to aberrant
ROS production.
98
While the exact mechanisms behind mtDNA content
regulation are still unclear, it seems changes in either direction
can be causative or indicative of disease.
99
Measurement of
mtDNA copy number can be done accurately by real-time PCR
methods, making this a useful approach for investigating the role
of mitochondrial metabolism in disease phenotypes.
Common mtDNA population variants
mtDNA variants accumulated over time differ between
population groups that have been separated for several thousand
years. Consequently, distinct lineages (mtDNA haplogroups) can
be drawn according to these sets of unique changes in mtDNA,
referred to as common population variants. The full human
mtDNA phylogeny can be accessed at
www.phylotree.org.
100
Much of the variation seen in modern humans is to be found
in the African haplogroups L0 to L6, but this variation has not
been as fully described as the variation on other continents.
European (e.g. I, J, K, H, T, U, V, W, X) and Asian (e.g. A, B, C,
D, F, G) haplogroups fall within super haplogroups M and N,
which in turn fall within L3.
mtDNA haplogroup association studies aim therefore to
associate these common mtDNA population variants with risk
for various complex diseases, such as diabetes, hypertension or
Parkinson’s disease.
101
mtDNA background has been shown to
correlate with the severity of cardiomyopathy caused by nDNA-
encoded mitochondrial protein mutations,
102
and increases the
penetrance of LHON-causing pathogenic mutations.
50,51
It has been proposed that mtDNA population variants could
contribute to the adaptability of population groups to their
environment by altering mitochondrial enzyme function.
103,104
By
analysing non-synonymous variants in 104 complete mtDNA
sequences from across the globe, Mishmar
et al.
103
found that
the
ATP6
and cytochrome
b
genes
were particularly variable
in arctic and temperate zones, respectively, leading them to
believe that positive selection had taken place. Stressors, such
as sudden changes in environment, could then influence the
degree of disease susceptibility of these environmentally adapted
population groups.
105
However, this hypothesis was contested by
others who have shown that there are significant differences in the
same measure in haplogroups from the same environment.
106,107
Additionally, Amo and Brand
108
put forward evidence to suggest
that certain bioenergetic parameters did not significantly differ
between mitochondria from arctic versus tropical haplogroups.
In contrast to the action of positive selection, the action of
negative or purifying selection on mtDNA has been established
for almost a decade.
107,109
One important point to consider is that
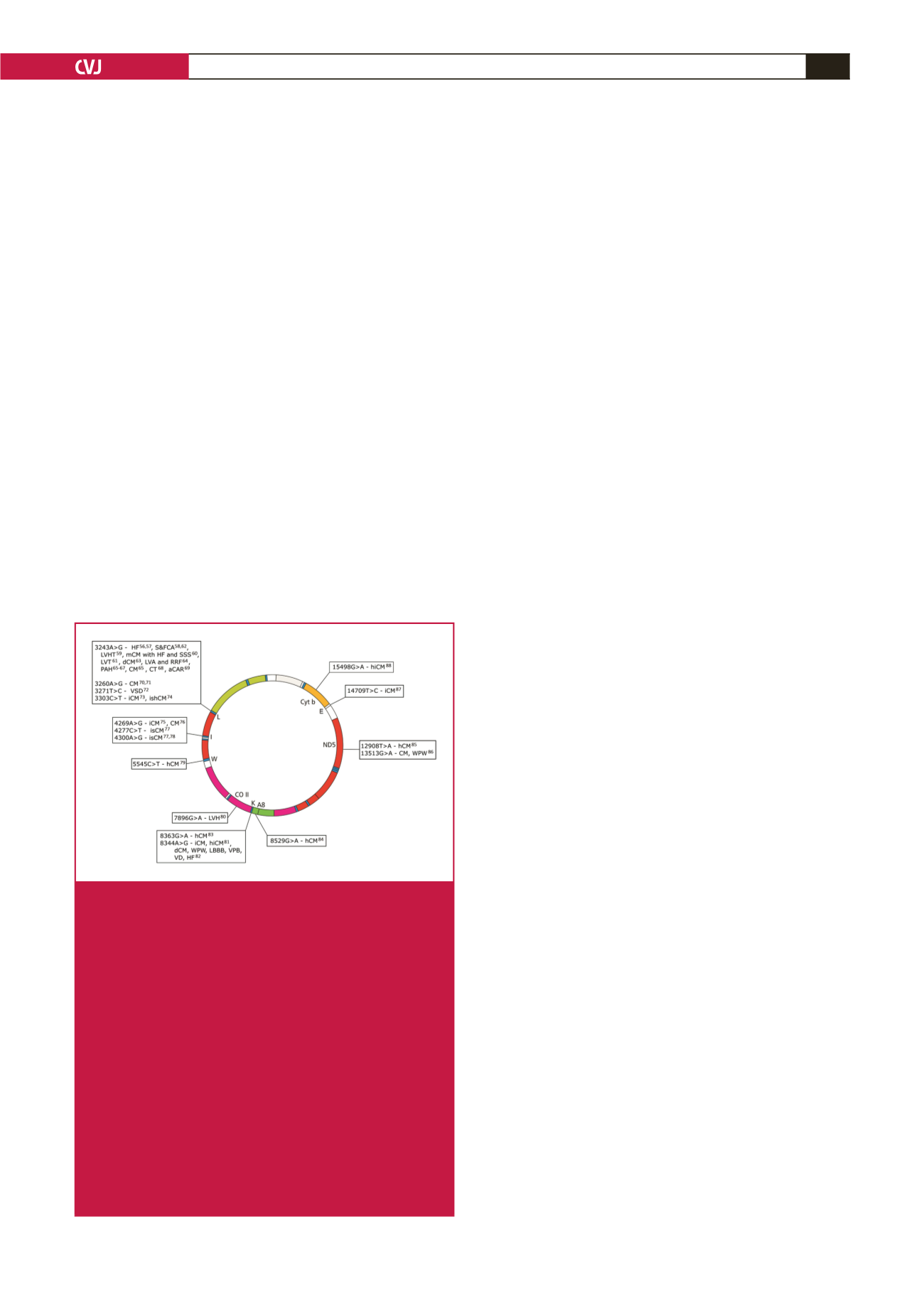
Fig. 3.
mtDNA morbidity map indicating clinically proven
mtDNA mutations that present with syndromic or isolat-
ed cardiac involvement. aCAR: abnormal cardiac auto-
nomic regulation; CM: cardiomyopathy; hCM: hyper-
trophic cardiomyopathy; dCM: dilated cardiomyopathy;
HF: heart failure; hiCM: histiocytoid cardiomyopathy;
iCM: infantile cardiomyopathy; ishCM: isolated hyper-
trophic cardiomyopathy; LBBB: left bundle branch block;
LVA: left ventricle abnormalities; LVH: left ventricular
hypertrophy; LVHT: left ventricular hyper-trabeculation/
non-compaction; mCM: mitochondrial cardiomyopathy;
PAH: pulmonary artery hypertension; RRF: ragged
red fibres; S&FCA: structural and functional cardiac
abnormality; SSS: sick sinus syndrome; VD: ventricular
dysfunction; VPB: ventricular premature beats; VSD:
ventricular septal defect; WPW: Wolff–Parkinson–White
syndrome. See Table 2 for a detailed list of mutations,
phenotype, references and pathogenicity scores, as
described in Mitchell
et al
.
44
and Yarham
et al
.
43

















