
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 31, No 2, March/April 2020
98
AFRICA
presence of obesity or obesity-associated metabolic features, and
the impact of adipocytokines relative to that of conventional
risk factors, have not been determined. In this regard, we show
that relationships between circulating resistin concentrations and
eGFR were unaffected by the extent of obesity, the presence of
obesity-associated metabolic features, or the metabolic syndrome
per se
and that the relative impact of resistin concentrations on
eGFR or CKD is at least as strong as modifiable conventional
risk factors.
Importantly, the combined impact of HOMA-IR and
circulating resistin concentrations on eGFR is substantially
greater than the combined impact of modifiable conventional
risk factors. As the relationships between resistin concentrations
and eGFR in the present study cannot be accounted for
by obesity or associated metabolic features, as with insulin
resistance, further studies are urgently required to identify the
origins of resistin beyond obesity in the South African context.
In this regard, as resistin in humans is derived largely from
circulating white blood cells, consideration must be given to the
possibility of chronic infective processes, such as that produced
by human immunodeficiency virus, contributing to this process.
Caution should be exercised in interpreting the results of this
study. The lack of independent relationships between several
adiposity indices and renal function in the present study, despite
our ability to show strong independent relationships with BP
and several metabolic parameters in the same sample as recently
described,
38
should not be interpreted to suggest that obesity
is not a cause of renal dysfunction in the population studied.
Indeed, there is a large body of evidence to show that both
insulin resistance and excess adiposity collectively contribute
to renal damage and to support a link between inflamed
adipose tissue and the development of kidney injury in obesity.
Moreover, a number of studies indicate that obesity elicits renal
dysfunction independent of insulin resistance.
However, there are several possible reasons why clinically
employed adiposity indices failed to show relationships
in the present study. First, obesity may be associated with
hyperfiltration and an increased GFR. Therefore eGFR may
underestimate the contribution of obesity to CKD. Nonetheless,
this would not explain a marked impact of insulin resistance
and resistin on eGFR, while adiposity indices failed to do so.
Second, in the present study we did not assess relationships
between visceral fat, which is often poorly indexed by indirect
measures such as waist circumference or waist-to-hip ratio,
and renal function. The fact that resistin was independently
associated with eGFR and that resistin is derived from white
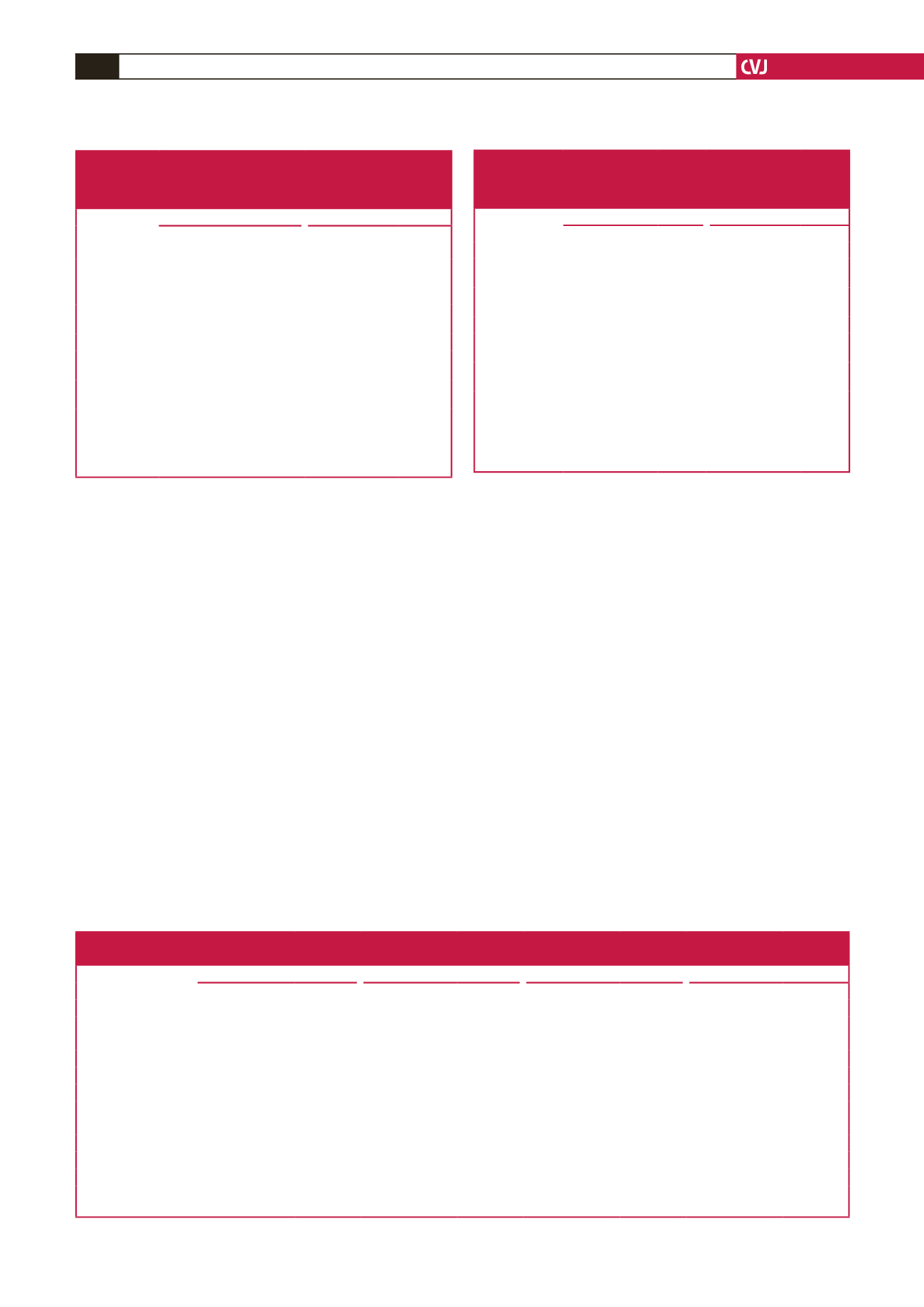
Table 6. Gender-specific multivariate-adjusted (partial
r
) relationships
between the homeostasis model of insulin resistance or resistin
concentrations and estimated glomerular filtration rate in non-diabetic
participants of a community sample and the full community sample
Women
Men
eGFR versus
Partial
r
(95% CI)
p
-value Partial
r
(95% CI)
p
-value
Non-diabetic participants
(
n
=
550)
(
n
=
322)
HOMA-IR
–0.177
(–0.258 to –0.093)
<
0.0001
–0.159
(–0.267 to –0.047)
<
0.005
Resistin
–0.133
(–0.216 to –0.048)
<
0.005
–0.144
(–0.252 to –0.032)
<
0.02
All participants
(
n
=
642)
(
n
=
368)
HOMA-IR
–0.159
(–0.235 to –0.080)
<
0.0005
–0.153
(–0.255 to –0.048)
<
0.005
Resistin
–0.152
(–0.228 to –0.073)
<
0.0005
–0.202
(–0.301 to –0.098)
<
0.0005
eGFR, estimated glomerular filtration rate; HOMA-IR, homeostasis model of
insulin resistance. Adjustments are for age, gender, conventional systolic blood
pressure, waist circumference, regular tobacco use, regular alcohol consumption,
diabetes mellitus (in all participants), HbA
1c
(in all participants) and the meta-
bolic syndrome.
Table 7. Impact of adjustments for C-reactive protein on multivariate
adjusted relationships between resistin concentrations and estimated
glomerular filtration rate in non-diabetic participants of a community
sample and the full community sample
MDRD eGFR
CKD-EPI eGFR
Resistin versus
Partial
r
(95% CI)
p
-value Partial
r
(95% CI)
p
-value
Non-diabetic participants
eGFR
–0.130
(–0.196 to –0.063)
<
0.0005
–0.129
(–0.195 to –0.062)
<
0.0005
+ CRP
–0.126
(–0.192 to –0.059)
<
0.0005
–0.125
(–0.191 to –0.058)
<
0.0005
All participants
eGFR
–0.160
(–0.221 to –0.097)
<
0.0001
–0.170
(–0.231 to –0.108)
<
0.0001
+ CRP
–0.152
(–0.214 to –0.090)
<
0.0001
–0.164
(–0.225 to –0.101)
<
0.0001
eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in
Renal Disease equation; CKD-EPI, Chronic Kidney Disease Epidemiology
equation. Adjustments are for age, conventional systolic blood pressure, waist
circumference, regular tobacco use, regular alcohol consumption, diabetes melli-
tus (in all participants), HbA
1c
(in all participants), the metabolic syndrome and
C-reactive protein as indicated.
Table 8. Relative impact [standardised slopes (
β
-coefficients)] of factors accounting for variations in estimated glomerular filtration rate in
non-diabetic participants of a community sample
Models with
Brachial SBP (n
=
850)
24-hour SBP (n
=
584)
Aortic SBP (n
=
843)
Aortic PWV (n
=
762)
eGFR versus
β
-coeff
±
SEM
p
-value
β
-coeff
±
SEM
p
-value
β
-coeff
±
SEM
p
-value
β
-coeff
±
SEM
p
-value
Age
–0.66
±
0.03
<
0.0001
–0.66
±
0.04
<
0.0001
–0.65
±
0.03
<
0.0001
–0.65
±
0.04
<
0.0001
HOMA-IR
–0.13
±
0.03
<
0.0001
–0.11
±
0.03
<
0.001
–0.12
±
0.03
<
0.0001
–0.13
±
0.03
<
0.0001
Resistin
–0.10
±
0.02
<
0.0001
–0.08
±
0.03
<
0.005
–0.11
±
0.02
<
0.0001
–0.11
±
0.03
<
0.0001
Hypertension
–0.001
±
0.033
0.99
–0.02
±
0.04
0.55
0.002
±
0.034
0.96
–0.01
±
0.03
0.73
Waist circumference
0.03
±
0.03
0.32
0.03
±
0.04
0.42
0.03
±
0.03
0.34
0.02
±
0.04
0.63
Glucose
–0.03
±
0.03
0.23
–0.05
±
0.04
0.19
–0.04
±
0.03
0.23
–0.04
±
0.03
0.16
Metabolic syndrome
–0.004
±
0.039
0.92
0.02
±
0.05
0.61
–0.003
±
0.039
0.94
0.04
±
0.04
0.36
Brachial SBP
–0.04
±
0.03
0.19
–
–
–
–
–
–
24-hour SBP
–
–
–0.04
±
0.03
0.23
–
–
–
–
Aortic SBP
–
–
–
–
–0.05
±
0.03
0.16
–
–
Aortic PWV
–
–
–
–
–
–
–0.07
±
0.03
<
0.05
eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; PWV, pulse-wave velocity;
β
-coeff, standardised
β
-coefficient; HOMA-IR, homeostasis model
of insulin resistance. Also included in the regression models were gender, regular tobacco use and regular alcohol consumption.



















